Abstract
The full-length extracellular domain (ECD) of protein tyrosine phosphatase δ (PTP-δ) functions as a ligand to promote cell adhesion and neurite outgrowth; this ECD contains 3 immunoglobulin (Ig) repeats and 8 fibronectin type III (FN III) repeats. However, it is not known which regions of the ECD regulate its ligand functions. Therefore, we constructed and expressed a fusion protein of the PTP-δ ECD lacking FN III repeats 4–8, and tested this protein for neuronal adhesion and neurite-promoting ability. Compared to the full-length isoform, the truncated ECD was poorer at promoting adhesion, but a more potent promoter of neurite growth. The results suggest that distal FN III repeats of PTP-δ are important in adhesive functions, but dispensable for neurite outgrowth promotion. As the predominant isoform of PTP-δ during neural development (type D) also lacks distal FN III repeats, the functional properties we observe may be relevant to periods of axon extension, suggesting that splice variants of receptor PTPs play distinct roles in neural development.
Keywords: Protein tyrosine phosphatase, axon growth, cell adhesion molecule, fibronectin type III repeat, immunoglobulin domain
Abbreviations: ECD, extracellular domain; FN III, fibronectin type III; Ig, immunoglobulin; PTP, protein tyrosine phosphatase; RPTP, receptor PTP
1. INTRODUCTION
Receptor protein tyrosine phosphatases (RPTPs) are a large family of transmembrane receptors that regulate neural development. Specifically, type IIa and type III RPTPs have been shown to regulate axon outgrowth during development and regeneration (Bixby 2000; Johnson and Van Vactor 2003). Interestingly, RPTPs can act in axon growth regulation not only as receptors, but also as ligands, through their various extracellular domains (ECDs) (Bixby 2000). Type IIa RPTPs are especially interesting in this regard, as their ECDs comprise immunoglobulin (Ig) and fibronectin type III (FN III) repeats, placing them in the Ig superfamily of cell adhesion molecules (CAMs).
We have focused on the type IIa RPTP PTP-δ, a homophilic CAM with an ECD comprising 3 Ig repeats and 8 FN III repeats (Figure 1A). When expressed as an Fc fusion protein, the full-length PTP-δ ECD is adhesive, promotes neurite outgrowth, and is an attractive guidance cue for forebrain neurons (Wang and Bixby 1999; Sun et al. 2000). In vivo studies suggest that PTP-δ is involved in the guidance and termination of both retinal and motor axons during embryonic development (Johnson and Van Vactor 2003; Stepanek et al. 2005; Uetani et al. 2006).
Figure 1.
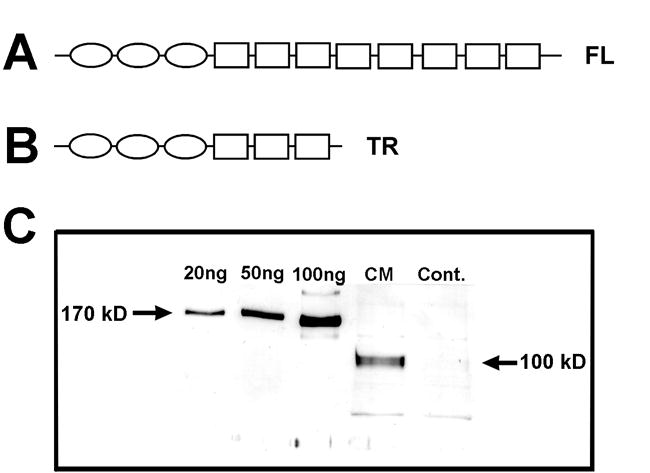
Expression of the truncated isoform of the PTP-δ ECD lacking the C-terminal FN III repeats 4–8. A. Domain structure of PTP-δ isoforms. The full-length (FL) ECD of PTP-δ (upper) contains 3 tandemly-linked immunoglobulin (Ig) repeats (ovals), followed by 8 FN III repeats (squares). B. The truncated (TR) construct (lower) that we produced is lacking the final 5 FN III repeats. C. Expression of the truncated fusion protein. The cDNA encoding the truncated ECD was fused in frame to the Fc portion of mouse IgG1, and the resulting fusion construct was expressed in stably-transformed CHO cells. Conditioned medium (CM) from one CHO cell line, and control CHO cell medium (Cont.) were separated on SDS-PAGE, and fusion proteins were identified by Western blotting using an anti-Fc antibody. An immunoreactive product of the expected size of the truncated fusion protein (100 kD) was seen in the conditioned medium. Amounts of secreted protein were estimated by comparison with known amounts of purified full-length PTP-δ fusion protein (170 kD band; left-hand lanes).
Several different PTP-δ isoforms are predicted to exist due to alternative splicing in the mouse and human; these are expressed in a tissue- and developmental stage-specific manner (Pulido et al. 1995a; Pulido et al. 1995b). Splice variants observed differ mainly in their ECD structure. For example, the truncated type D isoform, which lacks FN III repeats 4–7, is the predominant variant expressed in the embryonic and neonatal CNS (Mizuno et al. 1994). Because PTP-δ appears to play a significant role during embryonic neural development, it is important to understand the contribution of C-terminal FN III repeats to the activities of the PTP-δ ECD.
Experiments on other axon regulatory-CAMs have shown that specific regions of the ECD are required for neuronal adhesion and neurite outgrowth. For example, both specific Ig repeats and specific FN III repeats of CAMs such as L1 and NCAM have been implicated in adhesion and promotion of neurite growth (Frei et al. 1992; Stahlhut et al. 1997; Haspel and Grumet 2003).
Thus, it is known that the full-length ECD of PTP-δ functions as a ligand to promote cell adhesion and neurite outgrowth, and that other CAMs require specific regions of their ECD for these activities. However, the regions of the ECD of PTP-δ required for neuronal adhesion and neurite outgrowth are not known. Therefore, we have constructed and expressed an Fc fusion protein of the PTP-δ ECD lacking the C-terminal FN III repeats 4–8, and examined the protein for adhesive and neurite-promoting activities. In addition to elucidating the different functions for specific regions of the PTP-δ ECD, the activities of these distal FN III repeats can shed light on functions of developmentally-expressed isoforms of PTP-δ lacking these FN III domains.
2. EXPERIMENTAL PROCEDURES
2.1 Materials
Fertilized Leghorn eggs were purchased from SPAFAS (Preston, CT), and incubated at 38°C until use. Cell culture media were purchased from the University of Miami Sylvester Cancer Center Media Facility. The full-length PTP-δ ECD/Fc fusion protein was expressed and purified as described (Wang and Bixby 1999).
2.2 Construction of the truncated PTP-δ ECD cDNA
The truncated ECD cDNA was subcloned from the full-length human PTP-δ cDNA (Wang and Bixby 1999). An SpeI site in the PTP-δ ECD (nt 1986 of NM_130391) was used to clone a cDNA fragment encoding all 3 Ig and the first 3 FN III repeats (Figure 1B) in frame with the Fc portion of mouse IgG1 in the pcDNA 3.0 expression vector. The truncated PTP-δ ECD construct was transfected and expressed as a fusion protein in stably transfected CHO cell lines as previously described (Wang and Bixby 1999). After transfection, established cell lines were screened for expression by Western blot analysis of conditioned media using anti-Fc antibody (Figure1C). The truncated fusion protein was purified on an anti mIgG1-agarose column as described (Wang and Bixby 1999).
2.3 Neurite outgrowth assay
For the neurite outgrowth assay, a 35-mm cell culture dish was coated with nitrocellulose as described (Wang and Bixby 1999). Different concentrations of the purified truncated and full-length PTP-δ ECD fusion proteins, or poly-D-Lysine (PDL) as a control, were spotted (12–13 spots/dish) onto nitrocellulose-coated culture dishes. E7 chick forebrain neurons (3 X 105/dish) were prepared and cultured on these substrates for 16 hrs at 37°C as described (Wang and Bixby 1999). Neurite outgrowth was determined by the percentage of neurons with neurites within each substrate spot. A neurite was defined as a process at least 2X the diameter of the cell soma. Neurite lengths were measured from digital images using NIH Image; at least 100 neurites were measured for each condition in at least 4 independent experiments.
2.4 Cell Adhesion assays
Tissue culture dishes were prepared exactly as described for the neurite outgrowth assay, except that BSA (50 μg/ml) was used as a negative control, and PDL was a positive control. Forebrain neurons were prepared, plated (5 X 105/dish) and allowed to grow at 37°C for either 1.5 hrs (short-term assay) or 16 hrs (long-term assay). Plates were fixed and washed, and the number of adherent neurons was counted from phase contrast digital photographs. At least 150 neurons were counted for each condition in at least 3 independent experiments (2 hr assay), or at least 70 neurons in 3 different experiments (16 hr assay).
2.5 Statistical Analysis
The Igor program (Wavemetrics) was used to fit curves for EC50 estimates. Statistical significance was analyzed by t tests and two factor ANOVA analysis. P values of less than 0.05 were considered significant.
3. RESULTS
Expression of the truncated PTP-δ ECD lacking C-terminal FN III repeats
We have previously shown that the ECD of full-length PTP-δ has ligand activities on chick forebrain neurons, including the promotion of neuronal adhesion and neurite outgrowth (Wang and Bixby 1999). However, to examine potential ligand functions of the C-terminal FN III repeats of PTP-δ, a truncated fusion protein was made, which fused the 3 Ig and first 3 FN III repeats of the PTP-δ ECD (Figure 1B) in frame with the Fc region of mouse IgG1. Appropriate expression and secretion of the soluble fusion protein (expected size 100 kD) was confirmed by Western Blot (Figure 1C). The truncated fusion protein was affinity-purified and used as a substrate for forebrain neurons in assays for adhesion and neurite outgrowth.
The truncated PTP-δ ECD is less adhesive than the full-length protein
We first examined the ability of the truncated ECD to promote neuronal adhesion. In a short-term adhesion assay, neurons adhered well both to the truncated ECD and to the full-length PTP-δ ECD (Figure 3A). The truncated fusion protein was as potent in promotion of adhesion as the full-length protein (EC50, truncated= 104 nM; EC50 full-length=113 nM; Figure 3 and Wang and Bixby, 1999), suggesting that the protein folded correctly and bound to receptors of similar affinity. However, the maximal adhesion promoted by the truncated protein was significantly less than that seen with the full-length protein, as assessed by total number of adherent neurons on optimal substrate concentrations (p < 0.05). This result suggests that the truncated protein, lacking the C-terminal FN III repeats, is less adhesive than the full-length protein.
Figure 3.

Quantification of adhesion and neurite growth mediated by the truncated ECD of PTP-δ lacking the C-terminal FN III repeats. A. Forebrain neurons were cultured for 1.5 hrs on PDL, BSA, the full-length PTP-δ ECD at 300 nM (FL 300), or the truncated ECD of PTP-δ at various concentrations in nM (TR500, TR 200, TR 100, TR 50). The concentration of the full-length PTP-δ ECD used was optimal for adhesion (Wang and Bixby, 1999 and data not shown). The number of neurons adhering after fixation and washing was quantified for at least 4 experiments and plotted as mean ± SEM. Asterisk, significantly different from TR 500; p < 0.05. B. Forebrain neurons were cultured for 16 hrs on PDL, the full-length PTP-δ ECD at various concentrations in nM (FL 600, FL 300, FL 150, FL 75, FL 37), or the truncated ECD of PTP-δ at various concentrations in nM (TR 500, TR 200, TR 100, TR 50), and the percentage of neurons with neurites was quantified for at least 4 different cultures (600 nM FL data are from one culture in duplicate) and plotted as mean ± SEM. Data for the full-length PTP-δ ECD at 150, 75, and 37 nM are re-graphed from Wang and Bixby, 1999.
To test whether the adhesive differences seen in the short-term adhesion assay might be significant for longer-term neuronal attachment, we examined neuronal adhesion in a 16 hr assay. When these cultures were examined there were clearly fewer neurons present on the truncated PTP-δ ECD substrates compared to the full-length protein (Figures 2A, C). To quantify this result, the number of neurons adhering after 16 hrs was counted for optimal concentrations of the PTP-δ substrates. Significantly more neurons remained adherent to the full-length ECD compared to the truncated ECD (32.3 ± 4.6 neurons/field, full-length; 14.2 ± 1.9 neurons/field, truncated; p < 0.005). Adherent E7 neurons do not die in detectable numbers during the first 24 hrs of culture (Bixby and Jhabvala 1992; Wang and Bixby 1999, and unpublished observations), so this difference reflects de-adhesion of neurons over 16 hrs. Thus, the truncated protein, lacking the distal FN III repeats, is less adhesive than the full-length protein. These data suggest that the C-terminal FN III repeats function in promotion of neuronal adhesion.
Figure 2.
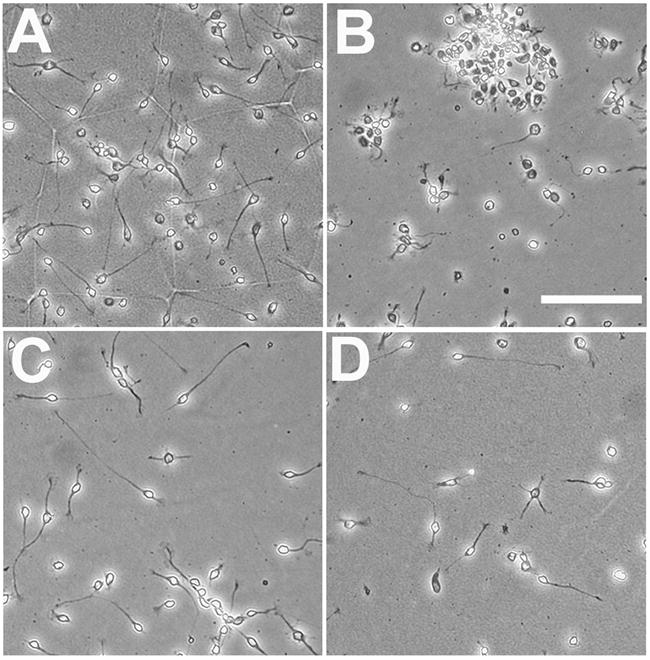
Growth of forebrain neurons on the truncated ECD of PTP-δ substrates lacking the C-terminal FN III repeats. Dissociated E7 chick forebrain neurons were plated on the full-length PTP-δ ECD (150 nM, A), poly-D-lysine (PDL, B), or the PTP-δ truncated ECD (200 nM, C; 100 nM, D), and cultured for 16 hrs prior to fixation. Neurons adhered as largely single cells to either isoform of the PTP-δ ECD, and grew long neurites in both cases. Although fewer neurons adhered to the truncated PTP-δ ECD substrate, neurites were equally long and numerous. Scale bar, 100 μm.
The truncated PTP-δ ECD is more potent and equally effective in neurite growth promotion
The ECD of full-length PTP-δ is a potent and effective promoter of neurite outgrowth (Wang and Bixby 1999). To determine whether this activity is influenced by the C-terminal FN III repeats, we performed neurite outgrowth experiments with the truncated PTP-δ ECD fusion protein, and compared growth with that promoted by the full-length ECD. Similar to the full-length ECD, the truncated ECD promoted vigorous neurite growth from forebrain neurons (Figures 2C, D); neurites were more numerous and longer than for cells grown for the same time on laminin substrates (Wang and Bixby, 1999, and data not shown). Quantitative analysis revealed that the percentage of neurons with neurites on the truncated PTP-δ ECD substrate was at least as high as for the full-length ECD (Figure 3B). The average length of individual neurites grown on optimal concentrations of the truncated ECD was also identical to that for neurons grown on optimal concentrations of the full-length ECD (38 ± 1.7 μm, full-length; 37 ± 1.6 μm, truncated; mean ± SEM). Interestingly, the potency of the truncated isoform for neurite growth promotion was about 3-fold higher than for the full-length protein (Estimated EC50, truncated= 56 nM; EC50 full-length=170 nM; Figure 3 and Wang and Bixby, 1999). Taken together, our results indicate that the PTP-δ ECD containing only FN III repeats 1–3 is more potent and equally effective in neurite growth promotion, compared to the full-length ECD.
4. DISCUSSION
The most important conclusion from this study is that the C-terminal FN III repeats excluded from the truncated PTP-δ ECD confer distinct ligand properties to the ECD of PTP-δ. Specifically, the construct tested in these assays, lacking FN-III repeats 4–8, is less adhesive and somewhat more growth promoting than the full-length variant. Elucidating the activities of the C-terminal FN III repeats might have implications for the regulatory roles of PTP-δ splice variants in neural development.
In the mouse, the type D isoform of PTP-δ, lacking distal FN III domains, is the only neural form expressed prior to P7, after which time the other 3 major isoforms (full-length, or type C, as well as types A and B) are all upregulated (Mizuno et al. 1994). If this isoform has properties similar to our truncated protein, the functional differences we observed in vitro may be relevant to in vivo activities. For example, it may be advantageous to increase adhesive activities relative to growth-promoting activities after the major phase of axon growth is over. Increasing evidence suggests that PTP-δ can regulate axon growth and guidance during development. Overexpression of a putative dominant negative isoform of PTP-δ inhibits growth of retinal axons in vivo (Johnson et al. 2001), RNAi-mediated knockdown of PTP-δ in motor neurons deranges guidance of motor axons (Stepanek et al. 2005), and null mutants of murine PTP-δ exhibit abnormal targeting of motor axons (Uetani et al. 2006). The PTP-δ ECD that we tested here differs slightly from the type D isoform in that it lacks the membrane-proximal FN III repeat (FN III repeat 8). It is possible that this repeat contributes to adhesive properties, but it is clearly not required for full promotion of neurite outgrowth.
The differential activities of the full-length (type C) ECD and the truncated ECD allow inferences concerning the functions of FN III repeats 4–7. Specifically, these FN III repeats are dispensable for promotion of neurite growth, but appear to be necessary for full neuronal adhesion. FN III repeats in other members of the immunoglobulin superfamily have been implicated in cell adhesion, as well as neurite outgrowth, heparin binding, and other protein interactions (Norenberg et al. 1996; Bennett et al. 1997; Adamsky et al. 2001; Koticha et al. 2005; Aricescu et al. 2006). It is clearly difficult to ascribe functions to FN III repeats as a class; rather each protein domain must be considered in its own context.
One suggestion of our findings is that there are distinct receptors responsible for different activities of the PTP-δ ECD. One receptor for PTP-δ is PTP-δ itself, in a homophilic interaction that would be predicted to mediate cell adhesion (Wang and Bixby, 1999). It could be that different activities result from interactions of the same proteins in different conformations or using distinct regions of the protein(s). This idea is illustrated by another member of the Ig superfamily, L1, which appears to cycle between folded and more linear conformations, and which binds different proteins using distinct modular domains (Brummendorf and Lemmon 2001; Haspel and Grumet 2003). It is notable that a number of distinct ligands and/or receptors have been identified for a close relative of PTP-δ, PTP-σ (Haj et al. 1999; Sajnani-Perez et al. 2003).
Our conclusions about the activities of the C-terminal FN III repeats of PTP-δ are based on analysis of glycosylated proteins produced in eukaryotic cells; these show the apparent molecular weight predicted from the native ECD. This point is noteworthy because conclusions about binding activities of other Ig superfamily CAMs differ, depending on whether bacterially-expressed or eukaryotically-expressed proteins are considered (Haspel and Grumet, 2003).
Our results are the first evidence that alternatively spliced isoforms of PTP-δ have distinct activities. Distinct activities of different isoforms are also likely for PTP-σ (Sajnani-Perez et al. 2003). In view of these findings, it will be important to examine activities of the type A isoform (with 1 Ig and 4 FN III repeats) and the type B isoform (with 1 Ig and 8 FN III repeats). PTP-δ, like its relative LAR (Zhang and Longo 1995), also has several mini-exons (Pulido et al. 1995a). In the case of LAR, such mini-exons can clearly be functionally important (Yang et al. 2003), and it will be worthwhile to test this for PTP-δ.
Our results demonstrate that the FN III repeats included in the developmentally expressed isoform of PTP-δ has distinct activities from those of its full-length counterpart. These differences implicate the C-terminal FN III repeats of PTP-δ in the regulation of neuronal adhesion, and suggest that developmentally regulated alternative splicing of PTP-δ mRNA has a functional significance.
Acknowledgments
We thank Jun Wang and Leilin Min for performing some of these experiments, and Dr. Vance Lemmon for reading the manuscript.
Footnotes
Supported by the Pediatric Scientist Developmental Program, NICHD Grant Award K12-HD00850-18 to MRG-B, and NIH R01 NS38920 to JLB
References
- Adamsky K, Schilling J, Garwood J, Faissner A, Peles E. Glial tumor cell adhesion is mediated by binding of the FNIII domain of receptor protein tyrosine phosphatase beta (RPTPbeta) to tenascin C. Oncogene. 2001;20:609–618. doi: 10.1038/sj.onc.1204119. [DOI] [PubMed] [Google Scholar]
- Aricescu AR, Hon WC, Siebold C, Lu W, van der Merwe PA, Jones EY. Molecular analysis of receptor protein tyrosine phosphatase mu-mediated cell adhesion. Embo J. 2006;25:701–712. doi: 10.1038/sj.emboj.7600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KL, Bradshaw J, Youngman T, Rodgers J, Greenfield B, Aruffo A, Linsley PS. Deleted in colorectal carcinoma (DCC) binds heparin via its fifth fibronectin type III domain. J Biol Chem. 1997;272:26940–26946. doi: 10.1074/jbc.272.43.26940. [DOI] [PubMed] [Google Scholar]
- Bixby JL. Receptor tyrosine phosphatases in axon growth and guidance. Neuroreport. 2000;11:R5–10. doi: 10.1097/00001756-200007140-00001. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Jhabvala P. Inhibition of tyrosine phosphorylation potentiates substrate-induced neurite growth. Journal of Neurobiology. 1992;23:468–480. doi: 10.1002/neu.480230503. [DOI] [PubMed] [Google Scholar]
- Brummendorf T, Lemmon V. Immunoglobulin superfamily receptors: cis-interactions, intracellular adapters and alternative splicing regulate adhesion. Curr Opin Cell Biol. 2001;13:611–618. doi: 10.1016/s0955-0674(00)00259-3. [DOI] [PubMed] [Google Scholar]
- Frei T, von Bohlen und Halbach F, Wille W, Schachner M. Different extracellular domains of the neural cell adhesion molecule (N-CAM) are involved in different functions. Journal of Cell Biology. 1992;118:177–194. doi: 10.1083/jcb.118.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj F, McKinnell I, Stoker A. Retinotectal ligands for the receptor tyrosine phosphatase CRYPalpha. Mol Cell Neurosci. 1999;14:225–240. doi: 10.1006/mcne.1999.0785. [DOI] [PubMed] [Google Scholar]
- Haspel J, Grumet M. The L1CAM extracellular region: a multi-domain protein with modular and cooperative binding modes. Front Biosci. 2003;8:s1210–1225. doi: 10.2741/1108. [DOI] [PubMed] [Google Scholar]
- Johnson KG, McKinnell IW, Stoker AW, Holt CE. Receptor protein tyrosine phosphatases regulate retinal ganglion cell axon outgrowth in the developing Xenopus visual system. J Neurobiol. 2001;49:99–117. doi: 10.1002/neu.1068. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- Koticha D, Babiarz J, Kane-Goldsmith N, Jacob J, Raju K, Grumet M. Cell adhesion and neurite outgrowth are promoted by neurofascin NF155 and inhibited by NF186. Mol Cell Neurosci. 2005;30:137–148. doi: 10.1016/j.mcn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Hasegawa K, Ogimoto M, Katagiri T, Yakura H. Developmental regulation of gene expression for the MPTP delta isoforms in the central nervous system and the immune system. FEBS Letters. 1994;355:223–228. doi: 10.1016/0014-5793(94)01188-5. [DOI] [PubMed] [Google Scholar]
- Norenberg U, Hubert M, Rathjen FG. Structural and functional characterization of tenascin-R (restrictin), an extracellular matrix glycoprotein of glial cells and neurons. Int J Dev Neurosci. 1996;14:217–231. doi: 10.1016/0736-5748(96)00009-3. [DOI] [PubMed] [Google Scholar]
- Pulido R, Krueger NX, Serra-Pages C, Saito H, Streuli M. Molecular characterization of the human transmembrane protein-tyrosine phosphatase delta. Evidence for tissue-specific expression of alternative human transmembrane protein-tyrosine phosphatase delta isoforms. Journal of Biological Chemistry. 1995a;270:6722–6728. doi: 10.1074/jbc.270.12.6722. [DOI] [PubMed] [Google Scholar]
- Pulido R, Serra-Pages C, Tang M, Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine- phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR- interacting protein LIP.1. Proc Natl Acad Sci U S A. 1995b;92:11686–11690. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajnani-Perez G, Chilton JK, Aricescu AR, Haj F, Stoker AW. Isoform-specific binding of the tyrosine phosphatase PTPsigma to a ligand in developing muscle. Mol Cell Neurosci. 2003;22:37–48. doi: 10.1016/s1044-7431(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Stahlhut M, Berezin V, Bock E, Ternaux JP. NCAM-fibronectin-type-III-domain substrata with and without a six-amino-acid-long proline-rich insert increase the dendritic and axonal arborization of spinal motoneurons. J Neurosci Res. 1997;48:112–121. [PubMed] [Google Scholar]
- Stepanek L, Stoker AW, Stoeckli E, Bixby JL. Receptor tyrosine phosphatases guide vertebrate motor axons during development. Journal of Neuroscience. 2005;25:3813–3823. doi: 10.1523/JNEUROSCI.4531-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QL, Wang J, Bookman RJ, Bixby JL. Growth Cone Steering by Receptor Tyrosine Phosphatase delta Defines a Distinct Class of Guidance Cue. Mol Cell Neurosci. 2000;16:686–695. doi: 10.1006/mcne.2000.0893. [DOI] [PubMed] [Google Scholar]
- Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bixby JL. Receptor tyrosine phosphatase-delta is a homophilic, neurite-promoting cell adhesion molecular for CNS neurons. Mol Cell Neurosci. 1999;14:370–384. doi: 10.1006/mcne.1999.0789. [DOI] [PubMed] [Google Scholar]
- Yang T, Bernabeu R, Xie Y, Zhang JS, Massa SM, Rempel HC, Longo FM. Leukocyte antigen-related protein tyrosine phosphatase receptor: a small ectodomain isoform functions as a homophilic ligand and promotes neurite outgrowth. J Neurosci. 2003;23:3353–3363. doi: 10.1523/JNEUROSCI.23-08-03353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Longo FM. LAR tyrosine phosphatase receptor: alternative splicing is preferential to the nervous system, coordinated with cell growth and generates novel isoforms containing extensive CAG repeats. Journal of Cell Biology. 1995;128:415–431. doi: 10.1083/jcb.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


