Abstract
Endonuclease V (endo V) recognizes a broad range of aberrations in DNA such as deaminated bases or mismatches. It nicks DNA at the second phosphodiester bond 3′ to a deaminated base or a mismatch. Endonuclease V obtained from Thermotoga maritima preferentially cleaves purine mismatches in certain sequence context. Endonuclease V has been combined with a high-fidelity DNA ligase to develop an enzymatic method for mutation scanning. A biochemical screening of site-directed mutants identified mutants in motifs III and IV that altered the base preferences in mismatch cleavage. Most profoundly, a single alanine substitution at Y80 position switched the enzyme to essentially a C-specific mismatch endonuclease, which recognized and cleaved A/C, C/A, T/C, C/T and even the previously refractory C/C mismatches. Y80A can also detect the G13D mutation in K-ras oncogene, an A/C mismatch embedded in a G/C rich sequence context that was previously inaccessible using the wild-type endo V. This investigation offers insights on base recognition and active site organization. Protein engineering in endo V may translate into better tools in mutation recognition and cancer mutation scanning.
INTRODUCTION
Techniques to scan unknown single nucleotide polymorphisms (SNPs) or point mutations are an essential tool in post-genomic era. Current mutation scanning methods include single-stranded conformational polymorphism (SSCP) and heteroduplex analysis (HA) (1,2), denaturing high performance liquid chromatography (DHPLC) (3), and chemical or enzymatic cleavage (4–7). Several enzymatic cleavage methods have been developed (7,8). T4 endonuclease VII and T7 endonuclease I, the two phage resolvases, have been used for mutation scanning with limited success due to high background generated by cleavage of non-mismatch sequences (9). Other enzymes such as MutY DNA glycosylase and thymine DNA glycosylase (TDG), and CEL1 nuclease have also been employed in mutation scanning (7,10).
Endonuclease V (endo V) is a DNA repair enzyme with unique enzymatic properties. Under physiological conditions, endo V cleaves deaminated bases at the second phosphodiester bond 3′ downstream to a lesion. By shifting reaction conditions to higher pH, metal cofactor to Mn2+, using excess enzyme, and/or using solvents such as dimethyl sulfoxide (DMSO) and betaine, this repertoire may be extended to include cleavage of most mismatched DNA base pairs (11–13). This enzymatic property has been explored for the development of mutation scanning techniques (8,14). We have devised a scheme that uses thermostable endo V obtained from Thermotoga maritima (Tma) to cleave mismatches and a high-fidelity thermostable DNA ligase from Thermus species AK16D to seal non-specific cleavage (8,15,16). Co-incubation of the two enzymes allows for endonucleolytic cleavage of mismatches with real-time resealing of matched nicks, allowing for detection of low-abundance mutations in tumor tissue at a ratio of 1:50 mutant to wild-type DNA (8,15).
Tma endo V preferentially cleaves purine bases in a mismatch in certain sequence context (13). The wild-type enzyme cleaves the C-containing mismatches the least and C/C mismatches are essentially resistant to cleavage (13). Even some A/C mismatches are refractory to cleavage when located in a G/C rich sequence context, as exemplified in the G13D mutation in K-ras (8). Identification of endo V variants that can cleave C-containing mismatches will broaden the applicability of the endo V/ligase mutation scanning technique. Although an endo V–DNA complex structure is not available, an extensive site-directed mutagenesis analysis has identified motifs and specific amino acid residues that influence base recognition and DNA–protein interactions (17). Taking advantage of a battery of over 60 endo V single-site mutants previously isolated, we screened for and identified endo V variants that possessed altered base preference in mismatch cleavage. Y80A in motif III converted endo V to essentially a C-specific mismatch cleavage variant that was capable of nicking refractory A/C mismatches in the K-ras gene.
MATERIALS AND METHODS
Materials
Purified deoxyribooligonucleotides were ordered from Integrated DNA Technologies Inc. (Coralville, IA). Duplex deoxyoligonucleotide substrates were prepared as described previously (17). The wild-type and mutant Tma endo V proteins and Tsp AK16D DNA ligase were purified as described previously (16–18).
Endo V cleavage assays
The cleavage reaction mixtures (10 μl) containing 10 mM HEPES-KOH (pH 7.4), 1 mM DTT, 2% glycerol, 5 mM MnCl2 unless otherwise specified, 10 nM oligonucleotide DNA substrate and 10 nM of Tma endo V protein unless otherwise specified were incubated at 65°C for 30 min. The reactions were terminated by the addition of an equal volume of GeneScan Stop Buffer [80% formamide, 50 mM EDTA (pH 8.0), and 1% blue dextran]. The reaction mixtures were then heated at 94°C for 3 min and cooled down on ice. Samples (3.5 μl) were loaded onto a 10% denaturing polyacrylamide gel containing 7 M urea. Electrophoresis was conducted at 1500 V for 1.5 h using an ABI 377 sequencer (Applied Biosystems). Cleavage products and remaining substrates were quantified using the GeneScan analysis software version 3.0.
PCR amplification of K-ras exon I
For detecting K-ras mutations, genomic DNA was extracted from cell lines as described (19). Cell lines HT 29 contains wild-type K-ras gene. SW480 contains G12V (G→T) mutation. DLD-1 contains G13D (G→A) mutation. K-ras exon I was amplified by PCR as described (8). To remove Taq DNA polymerase, 4 μl of 20 mg/ml proteinase K (Qiagen) was added to the PCR mixtures (50 μl) and incubated at 70°C for 10 min. Proteinase K was inactivated by incubating at 80°C for 10 min. Amplicons containing wild-type sequence were added in approximately equal ratios when missing from the sample (i.e. pure mutant cell line DNA). The mixed PCR fragments, were heated at 94°C for 1 min to denature the DNA, and then cooled at 65°C for 15 min and at room temperature for 15 min to allow efficient formation of heteroduplex DNA.
To generate sticky ended PCR products, K-ras exon I was amplified as described with the exception that the PCR primers are as follows (8): Oligo 1, 5′-CCCCGCTGAGGATAGTGTATTAACCTTATGTGTGACATGTTC-3′ (underlined: N.BbvC IA site); Oligo 2, 5′-FAM-CCCCCCTCAGCAAAATGGTCAGAGAAACCTTTATCTGTATC-3′ (underlined: N.BbvC IB site, which is complementary to the N.BbvC IA site). After PCR, the top strand contained two N.BbvC IA sites and the bottom strand contained two N.BbvC IB sites (Figure 4A). Post-PCR processing and formation of duplex DNA were carried out as described above. PCR products (6 μg) were then digested at 37°C overnight with 60 units of N.BbvC IA in NEBuffer 2 (New England Biolabs). The reaction mixtures were extracted with phenol/chloroform/isoamyl alcohol (25:24:1) to remove proteins and passed through microcon YM-50 spin column (Millipore) to remove the small DNA fragments generated by BbvC IA nicking.
RESULTS AND DISCUSSION
Examination of base preferences of mismatch cleavage in endonuclease V mutants
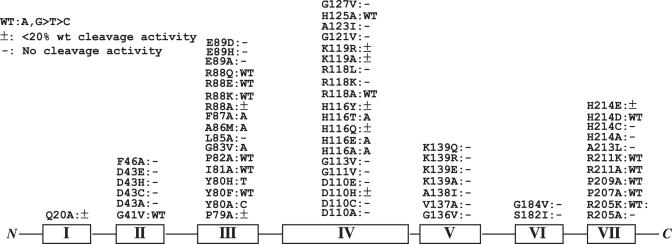
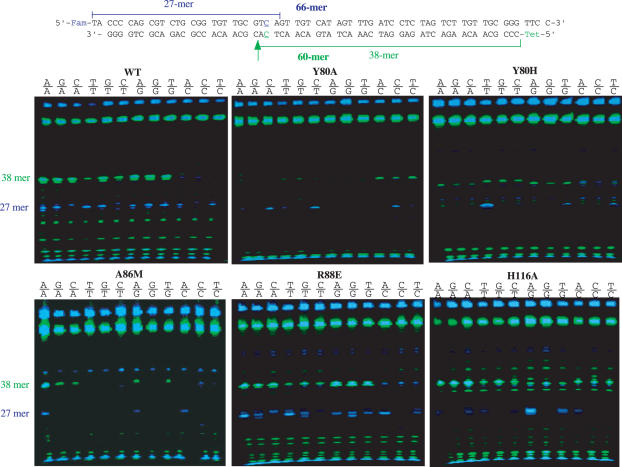
Endonuclease V contains seven conserved motifs in which motifs III and IV play a major role in protein–DNA interactions (17). We screened a total of 64 mutants previously isolated for mismatch cleavage activity (Figure 1) (17). The assays were performed in the presence of Mn2+ instead of Mg2+ since endo V enzymes show enhanced mismatch cleavage with Mn2+ (12,13). As expected, a majority of mutants lost mismatch cleavage activity. Other mutants still maintained mismatch cleavage activity in a pattern similar to the wild-type enzyme, which included G41V in motif II; Y80F, I81A, P82A, R88E, R88K and R88Q in motif III, R118A and H125A in motif IV; R205K, P207A, P209A, R211A, R211K and H214D in motif VII (Figure 1). Yet, several mutants in motifs III and IV showed quite distinctively altered base preference in mismatch cleavage. An alanine substitution at Y80 position essentially switches the base preference from purine mismatches to C-specific mismatches (Figures 1 and 2). All five C-containing mismatches were cleaved by Y80A (Figure 2, compare the band intensities in wild-type and Y80A). Most remarkably, even the refractory C/C mismatch in this sequence context was cleaved on both strands (Figure 2, C/C lane in Y80A). On the other hand, cleavage of other mismatches was minimum or not detected. A histidine substitution at Y80 rendered the enzyme more active in cleaving T-containing mismatches, while reducing the cleavage of other mismatches (Figure 2, Y80H). Apparently, A86M preferentially cleaved A-containing mismatches (Figure 2, A86M). All four A-containing strands G/A, C/A, A/G and A/C and both strands in A/A were cleaved by A86M. Other mutants such as G83V and F87A also showed preference for A bases (Figure 1). The base preference in R88E remained similar to the wild-type enzyme, i.e. G and A bases were preferred. However, the cleavage site on the top strand (blue band) is more promiscuous. Cleavage at 1 nt closer or 1 nt further away from the mismatches was observed (Figure 2, R88E). Similar cleavage site promiscuity occurred in R88Q (data not shown). A few H116 mutants such as H116A, H116E and H116T somewhat preferred the A base in a mismatch (Figures 1 and 2).
Figure 1.
Base preference of mismatch cleavage of Tma endonuclease V mutants. Cleavage reactions were performed as described in Materials and Methods. Motifs are shown in Roman letters. See (17) for sequence alignment.
Figure 2.
Representative GeneScan gel pictures of mismatch cleavage. Cleavage reactions were performed as described in Materials and Methods.
Cleavage of A/C mismatches in synthetic K-ras substrates
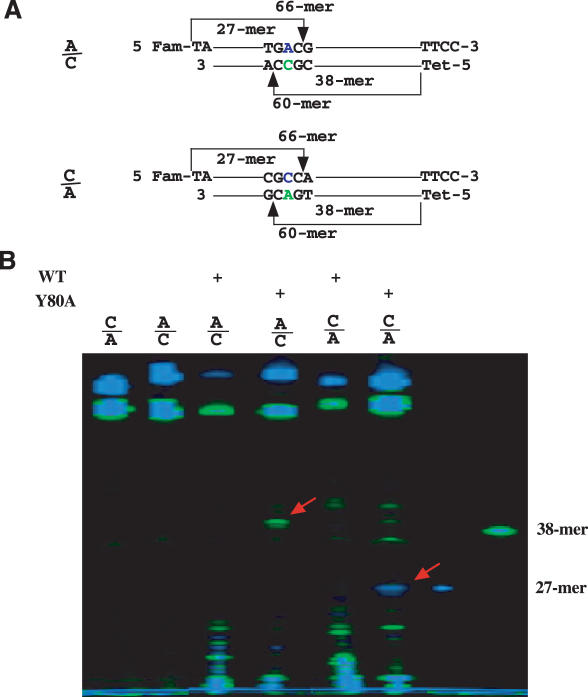
Given the strong preference of Y80A for the C base in a mismatch, we tested its ability to cleave C-containing mismatches that were refractory for the wild-type enzyme. Previously, we developed an enzymatic mutation scanning method, which takes advantage of the mismatch cleavage of endo V and nicking joining activity of DNA ligase to seal non-specific cleavage at matched bases (8). During the course of that study, we found G13D mutation in K-ras was completely refractory to endo V cleavage when using Mg2+ as cofactor in the presence of both 5% (V/V) DMSO and 1.5 M betaine. G13D is a G to A transition that yields G/T and A/C mismatches. A closer look at the flanking sequence indicates that the mismatches are located in a G/C rich sequence context (TGGCG, the mutation site is underlined), which may make it difficult for endo V to cleave (11). To test the ability of Y80A to cleave this sequence, we synthesized oligodeoxynucleotide substrate that was identical to the G13D sequence in K-ras (Figure 3A, A/C). The overall design was consistent with the mismatch substrates used for initial activity screening (Figure 2). When using Mn2+ as the metal cofactor, the wild-type endo V exhibited non-specific fragmentation of both the top and the bottom strand as a result of non-specific cleavage, but did not yield correct length fragments from the mismatched base pair (Figure 3B). Remarkably, Y80A generated a cleavage band from the bottom C-containing strand at ∼38–39mer position, indicating that the altered base preference has enabled the mutant to cleave the refractory sequence (Figure 3B). To verify the specificity of the cleavage by Y80A, we synthesized a similar substrate but with the C base on the top strand, which would generate a 27mer if cleaved (Figure 3A, C/A). Again, the Y80A cleaved the C-containing strand in the C/A mismatch at the anticipated position, while the wild-type enzyme generated lower molecular weight fragments (Figure 3B). These results confirmed the C base preference of the Y80A mutant in the refractory sequence.
Figure 3.
Cleavage of A/C mismatch in synthetic K-ras G13D sequence by Y80A Tma endonuclease V mutant. Cleavage reactions were performed as described in Materials and Methods with 2.5 mM MnCl2. (A) Schematic illustration of A/C cleavage. A/C heteroduplex was formed by annealing of 5′-FAM-TAACTTGTGGTAG TTGGAGCTGGTGACGTAGGCAAGAGTGCCTTGACGATACAGCTAATTCATTCC-3′ and 5′-TET-TGAATTAGCTGTAGCGTCAAGGCACTCTTGCCTACGCCACCA GCTCCAACTACCACAAGT-3′. C/A heteroduplex was formed by annealing of 5′-FAM-TATCGTCAAGGCACTCTTGCCTA CGCCACCAGCTCCAACTACCACAAGTTTATATTCAGTCATTCC-3′ and 5′-TET-TGACTGATAATAAA CTTGTGGTAGTTGGAGCTGGTGACGTAGGCAAGAGTGCCTTGACGA-3′. (B) Cleavage of A/C K-ras G13D mismatch by wild-type Tma endo V and Y80A mutant.
Cleavage of A/C mismatch in K-ras amplicons
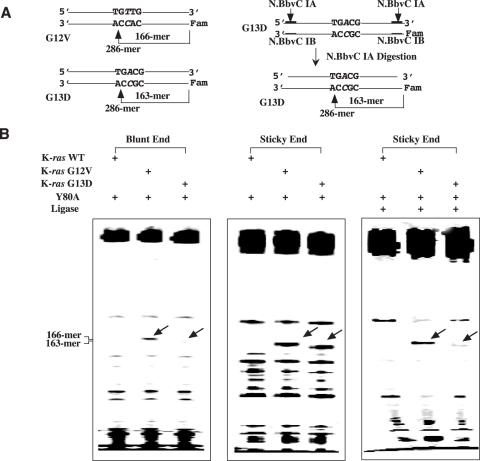
To test the ability of the Y80A mutant to cleave PCR products, we amplified the exon 1 of the K-ras gene from both the wild-type, G12V and G13D mutant cell lines. Heteroduplexes were generated by mixing the wild-type PCR amplicon with the mutant amplicons (Figure 4A, left). The 286 bp long heteroduplexes containing T/C and G/A mismatches from G12V and A/C and G/T mismatches from G13D were treated with Y80A mutant endo V. Since Y80A acted as a C-specific mismatch endonuclease (Figures 2 and 3), we scored the specific cleavage bands as resulting from cleaving C-containing mismatches. As expected, G12V was cleaved by Y80A on the C-containing strand to yield a 166mer product (Figure 4B, left). However, cleavage of A/C mismatch in the G13D was minimal (Figure 4B, left). Previously, we have observed a reduction in fluorescence signal in blunt end amplicons due to cleavage of the fluorescent label and the adjacent base by endo V, liberating the label from the amplicon (15). We suspected similar cleavage event might have occurred in the blunt ended amplicons that have reduced the cleavage product signals (Figure 4B left, bottom of gel). Given that the synthetic duplexes contained overhangs (Figure 3), we thought the overhangs at the ends may reduce the loss of fluorescence signal by endo V. We, therefore, designed a method to convert the PCR amplicons to sticky ended duplexes (Figure 4A, right). N.BbvC IA recognizes double-stranded 5′-GC↓TGAGG-3′ sequence and nicks between the C and T. The recognition sequence was incorporated into the PCR primers for amplifying the exon I of K-ras gene (see Materials and Methods for details). The resulting PCR amplicons were then treated with N.BbvC IA to generate a two-base overhang at the 3′ end and five-base overhang at the 5′ end for the C-containing strand, respectively (Figure 4A, right). Both the G12V and G13D heteroduplexes were cleaved by Y80A mutant endo V (Figure 4B, middle). The non-specific products were sealed by the high-fidelity Tsp. AK16D ligase, thus reducing the background (Figure 4B, right). Some of the mismatch cleavage products were also sealed by the DNA ligase (16), resulting in a reduction in the intensity of the specific band.
Figure 4.
Cleavage of A/C mismatch in K-ras G13D sequence amplified from colon cancer cell lines by Tma endo V mutant Y80A. (A) Schematic illustration of blunt end and sticky end heteroduplex G12V and G13D PCR products. See Materials and Methods for details. (B) Cleavage of G13D by Tma endo V mutant Y80A. Cleavage reaction mixtures (10 μl) containing 10 mM HEPES-KOH (pH 7.4), 1 mM DTT, 2% glycerol, 2.5 mM MnCl2, 100 ng of wild-type K-ras homoduplex or G12V heteroduplex or G13D heteroduplex and 100 nM Tma endo V mutant Y80A protein were incubated at 65°C for 30 min. For the reactions that were followed by ligation, the amount of K-ras homoduplex or heteroduplex was increased to 200 ng in the cleavage reactions. The cleavage reaction mixtures were filtered through an YM-10 microcon spin column and washed with TE buffer containing 10 mM Tris–HCl (pH 7.6) and 1 mM EDTA. To seal the non-specific nicks, the washed cleavage reaction mixtures (in 6 μl TE) were supplemented with 1 μl of 10 × Taklig buffer [20 mM Tris–HCl (pH 7.6), 100 mM KCl, 10 mM DTT, 20 μg/ml BSA], 1 μl of 100 mM MgCl2, 1 μl of 10 mM NAD+ and 1 μl of 20 nM Tsp AK16D ligase. The ligation mixtures were incubated at 65°C for 20 min.
This work identified endo V variant enzymes with substantially altered base preferences in mismatch cleavage. Since all these variant enzymes contained changes in motifs III and IV, this underscores the important role these motifs play in base recognition (Figure 1). Consistent with a previous study (17), Y80 and H116 appear to be important determinants of base recognition. Although an endo V–DNA co-crystal structure is not available, secondary structure analysis indicates that both Y80 and H116 are located in loop regions (20). We speculate that motifs III and IV are components of recognition loops that are involved in specific base recognition.
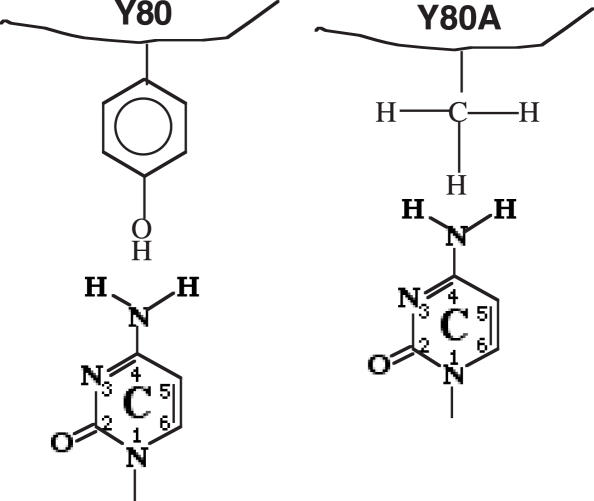
Y80A is the most striking in that it essentially converts the enzyme to a C-specific mismatch endonuclease (Figure 2). Consequently, the previous refractory C/C mismatch for the wild-type enzyme now becomes cleavable by the Y80A mutant. First, how does a single alanine substitution at Y80 position accomplish such a dramatic alteration in base preference? A simple model is illustrated in Figure 5. In the wild-type enzyme, Y80 imposes an unfavorable interaction with a C base, in which the amino group at C4 position spatially clashes with the bulky tyrosine residue. This steric hindrance prevents the wild-type endo V from recognizing and cleaving C-containing strand in a mismatch. By substituting the phenol side chain with a small methyl group, Y80A releases the steric tension and allows the C base to be accommodated in the recognition pocket (Figure 5). A comparison with uracil DNA glycosylase (UDG) is illuminating. The N204 in the recognition site of human UDG forms hydrogen bonds with O4 and N3 of uracil via the amide side chain and the Y147 excludes a thymine base by steric complementarity (21). Interestingly, N204D confers cytosine DNA glycosylase to hUDG by forming hydrogen bonds with the C4-amino group and the N3-nitrogen via the carboxyl side chain, while Y147A switches the enzyme to TDG by preventing the steric clash with the C5-methyl group of the thymine base (22). It is possible that endo V and UDG adopted a similar strategy as part of base-specific recognition mechanism (22–26).
Figure 5.
A hypothetical model for alteration of base recognition by Y80A. See text for details.
The surprising alteration in base preference of mismatch cleavage prompted us to investigate the potential implication in improving the endo V/ligase mutation scanning technique previously reported (8). The use of this technique in scanning K-ras mutations met with difficulty partly due to the inability of the wild-type endo V to cleave A/C mismatches in some G/C rich sequence context (8). Data presented here indicate that the Y80A is not only specific for C-containing mismatches, but also for those embedded in G/C rich environment (Figure 3). Therefore, the C-specific mismatch cleavage ability may have enabled the Y80A to recognize and nick the C-strand previously not accessible by the wt endo V. Based on the model explained above, favorable interactions between Y80A and a C base may facilitate the base recognition process, which assists in guiding the complex to a catalytically competent path. Likewise, the previously inaccessible C/C mismatch now becomes a substrate for Y80A (Figure 2). The difference in A/C mismatch cleavage efficiency between amplicons with blunt or overhang ends is due to loss of fluorescence signal by endo V cleavage. This problem was previously addressed by synthesizing modified primers that are refractory to endo V cleavage (15). Introducing a nicking site into a PCR primer provides a simple alternative method to maintain mismatch cleavage signal. This study demonstrates how malleable endo V is, allowing for alteration of base preference in mismatch cleavage by single amino acid changes. Some of these mutants offer the potential for developing base-specific endo V/DNA ligase mutation scanning assays.
Acknowledgments
We thank Dr. Pu-Chun Ke for critically reading the manuscript and members of Cao and Barany labs for stimulating discussions. This project was supported in the Cao lab in part by CSREES/USDA (SC-1700274, technical contribution No. 5189), the NIH (GM 067744), the Concern Foundation and DOD-Army Research Office (W911NF-05-1-0335). Work in the Barany lab was supported by the NCI (P01 CA65930-08). Funding to pay the Open Access publication charges for this article was provided by DOD.
Conflict of interest statement. None declared.
REFERENCES
- 1.Highsmith W.E., Jr, Jin Q., Nataraj A.J., O'Connor J.M., Burland V.D., Baubonis W.R., Curtis F.P., Kusukawa N., Garner M.M. Use of a DNA toolbox for the characterization of mutation scanning methods. I: construction of the toolbox and evaluation of heteroduplex analysis. Electrophoresis. 1999;20:1186–1194. doi: 10.1002/(SICI)1522-2683(19990101)20:6<1186::AID-ELPS1186>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Highsmith W.E., Jr, Nataraj A.J., Jin Q., O'Connor J.M., El-Nabi S.H., Kusukawa N., Garner M.M. Use of DNA toolbox for the characterization of mutation scanning methods. II: evaluation of single-strand conformation polymorphism analysis. Electrophoresis. 1999;20:1195–1203. doi: 10.1002/(SICI)1522-2683(19990101)20:6<1195::AID-ELPS1195>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Hecker K.H., Taylor P.D., Gjerde D.T. Mutation detection by denaturing DNA chromatography using fluorescently labeled polymerase chain reaction products. Anal. Biochem. 1999;272:156–164. doi: 10.1006/abio.1999.4171. [DOI] [PubMed] [Google Scholar]
- 4.Bui C.T., Lambrinakos A., Babon J.J., Cotton R.G. Chemical cleavage reactions of DNA on solid support: application in mutation detection. BMC Chem. Biol. 2003;3:1. doi: 10.1186/1472-6769-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babon J.J., McKenzie M., Cotton R.G. The use of resolvases T4 endonuclease VII and T7 endonuclease I in mutation detection. Mol. Biotechnol. 2003;23:73–81. doi: 10.1385/MB:23:1:73. [DOI] [PubMed] [Google Scholar]
- 6.Glare E.M., Jenkins A.J., Cotton R.G. Plastic contaminant masquerades as DNA in mutation detection by denaturing HPLC. Biotechniques. 2003;34:59–60. doi: 10.2144/03341bm09. [DOI] [PubMed] [Google Scholar]
- 7.Yeung A.T., Hattangadi D., Blakesley L., Nicolas E. Enzymatic mutation detection technologies. Biotechniques. 2005;38:749–758. doi: 10.2144/05385RV01. [DOI] [PubMed] [Google Scholar]
- 8.Huang J., Kirk B., Favis R., Soussi T., Paty P., Cao W., Barany F. An endonuclease/ligase based mutation scanning method especially suited for analysis of neoplastic tissue. Oncogene. 2002;21:1909–1921. doi: 10.1038/sj.onc.1205109. [DOI] [PubMed] [Google Scholar]
- 9.Mashal R.D., Koontz J., Sklar J. Detection of mutations by cleavage of DNA heteroduplexes with bacteriophage resolvases. Nature Genet. 1995;9:177–183. doi: 10.1038/ng0295-177. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Kaur M., Price B.D., Tetradis S., Makrigiorgos G.M. An amplification and ligation-based method to scan for unknown mutations in DNA. Hum. Mutat. 2002;20:139–147. doi: 10.1002/humu.10106. [DOI] [PubMed] [Google Scholar]
- 11.Yao M., Kow Y.W. Strand-specific cleavage of mismatch-containing DNA by deoxyinosine 3′-endonuclease from Escherichia coli. J. Biol. Chem. 1994;269:31390–31396. [PubMed] [Google Scholar]
- 12.Feng H., Klutz A.M., Cao W. Active site plasticity of endonuclease V from Salmonella typhimurium. Biochemistry. 2005;44:675–683. doi: 10.1021/bi048752j. [DOI] [PubMed] [Google Scholar]
- 13.Huang J., Lu J., Barany F., Cao W. Multiple cleavage activities of endonuclease V from Thermotoga maritima: recognition and strand nicking mechanism. Biochemistry. 2001;40:8738–8748. doi: 10.1021/bi010183h. [DOI] [PubMed] [Google Scholar]
- 14.Bazar L., Collier G., Vanek P., Siles B., Kow Y., Doetsch P., Cunningham R., Chirikjian J. Mutation identification DNA analysis system (MIDAS) for detection of known mutations. Electrophoresis. 1999;20:1141–1148. doi: 10.1002/(SICI)1522-2683(19990101)20:6<1141::AID-ELPS1141>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Pincas H., Pingle M.R., Huang J., Lao K., Paty P.B., Friedman A.M., Barany F. High sensitivity EndoV mutation scanning through real-time ligase proofreading. Nucleic Acids Res. 2004;32:e148. doi: 10.1093/nar/gnh150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong J., Cao W., Barany F. Biochemical properties of a high fidelity DNA ligase from Thermus species AK16D. Nucleic Acids Res. 1999;27:788–794. doi: 10.1093/nar/27.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng H., Dong L., Klutz A.M., Aghaebrahim N., Cao W. Defining amino acid residues involved in DNA–protein interactions and revelation of 3′-exonuclease activity in endonuclease V. Biochemistry. 2005;44:11486–11495. doi: 10.1021/bi050837c. [DOI] [PubMed] [Google Scholar]
- 18.Huang J., Lu J., Barany F., Cao W. Mutational analysis of endonuclease V from Thermotoga maritima. Biochemistry. 2002;41:8342–8350. doi: 10.1021/bi015960s. [DOI] [PubMed] [Google Scholar]
- 19.Khanna M., Park P., Zirvi M., Cao W., Picon A., Day J., Paty P., Barany F. Multiplex PCR/LDR for detection of K-ras mutations in primary colon tumors. Oncogene. 1999;18:27–38. doi: 10.1038/sj.onc.1202291. [DOI] [PubMed] [Google Scholar]
- 20.Rand T.A., Ginalski K., Grishin N.V., Wang X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc. Natl Acad. Sci. USA. 2004;101:14385–14389. doi: 10.1073/pnas.0405913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mol C.D., Arvai A.S., Slupphaug G., Kavli B., Alseth I., Krokan H.E., Tainer J.A. Crystal structure and mutational analysis of human uracil-DNA glycosylase: structural basis for specificity and catalysis. Cell. 1995;80:869–878. doi: 10.1016/0092-8674(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 22.Kavli B., Slupphaug G., Mol C.D., Arvai A.S., Peterson S.B., Tainer J.A., Krokan H.E. Excision of cytosine and thymine from DNA by mutants of human uracil-DNA glycosylase. EMBO J. 1996;15:3442–3447. [PMC free article] [PubMed] [Google Scholar]
- 23.Handa P., Acharya N., Varshney U. Effects of mutations at tyrosine 66 and asparagine 123 in the active site pocket of Escherichia coli uracil DNA glycosylase on uracil excision from synthetic DNA oligomers: evidence for the occurrence of long-range interactions between the enzyme and substrate. Nucleic Acids Res. 2002;30:3086–3095. doi: 10.1093/nar/gkf425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savva R., McAuley-Hecht K., Brown T., Pearl L. The structural basis of specific base-excision repair by uracil-DNA glycosylase. Nature. 1995;373:487–493. doi: 10.1038/373487a0. [DOI] [PubMed] [Google Scholar]
- 25.Kwon K., Jiang Y.L., Stivers J.T. Rational engineering of a DNA glycosylase specific for an unnatural cytosine:pyrene base pair. Chem. Biol. 2003;10:351–359. doi: 10.1016/s1074-5521(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 26.Hitchcock T.M., Gao H., Cao W. Cleavage of deoxyoxanosine-containing oligodeoxyribonucleotides by bacterial endonuclease V. Nucleic Acids Res. 2004;32:4071–4080. doi: 10.1093/nar/gkh747. [DOI] [PMC free article] [PubMed] [Google Scholar]