Abstract
Previous research has demonstrated that rats reared in an enriched condition (EC) with novel objects and social partners self-administer less amphetamine compared to rats raised in an isolated condition (IC). However, it is unclear if the enrichment-induced decrease in stimulant self-administration generalizes to non-drug rewards such as those provided by novel environmental stimuli. In the current study, EC, IC, and social condition (SC) rats were raised from 21 to 51 days of age before being tested in a two-lever operant conditioning chamber in which responding on one lever (active lever) resulted in illumination of a cue light. In Experiment 1, rats were initially assessed for baseline responding (no contingency) and then the contingent light was introduced. EC rats responded less than IC rats for the contingent light stimulus; however, EC rats also displayed a lower rate of baseline responding. In Experiment 2, rats were trained initially to lever press for a sucrose reward to decrease differences in baseline responding. While sucrose pretraining decreased baseline response differences between groups, EC rats still responded less for the contingent light stimulus than IC or SC rats. These results suggest that environmental enrichment decreases the incentive value of visual novelty.
Keywords: Enrichment, Impoverished, Novelty, Operant, Light
It has been hypothesized that exposure to novel stimuli and exposure to drugs of abuse activate, at least in part, a common mesolimbic dopamine reward system (Bardo et al., 1996). Similar to drugs of abuse, exposure to novel stimuli increases levels of extracellular dopamine and its metabolites in the medial pre-frontal cortex (Feenstra and Botterblom, 1996; Beaufour et al., 2001). When a rat enters a novel compartment from a familiar compartment, there is a transient and rapid surge in accumbal dopamine release recorded by in vivo voltammetry (Rebec et al., 1997). Further, injections of the neurotoxin 6-hydroxydopamine (6-OHDA) into the mesolimbic DA system disrupt the increase in locomotion and rearing normally elicited by novel stimuli (Fink and Smith, 1979; Mogenson and Nielsen, 1984).
While genetic factors contribute to an individual’s response to novelty (Oliverio and Messeri, 1973; Peeler and Nowakowski, 1987; Crusio et al., 1989), environmental factors also contribute. One environmental factor that alters an individual’s response to novelty is the amount of sensory stimulation experienced during development. A widely used method that alters novelty during development is the environmental enrichment paradigm (Renner and Rosenzweig, 1987). In this paradigm, rats are housed for several weeks with either social cohorts and novel objects in an enriched condition (EC) or individually without objects in an isolated condition (IC). EC rats subsequently display less activity than IC rats in an inescapable novel environment (Lore and Levowitz, 1966; Fuller, 1967; Bowling et al., 1993; Smith et al., 1997; Green et al., 2003). EC rats also approach novelty more quickly than IC rats in a free-choice test (Fuller, 1967; Renner and Rosenzweig, 1986; Widman and Rosellini, 1990), suggesting that EC rats may find novel stimuli to be less stressful. However, while they approach novelty more quickly, EC rats habituate to novel stimuli more rapidly with repeated exposure (Zimmermann et al., 2001), suggesting that the relative incentive value of novelty may be reduced in EC rats.
Consistent with the effects of environmental enrichment on novelty-seeking, enrichment decreases the relative incentive value of psychostimulant drugs when assessed with operant procedures. For example, EC rats self-administer less amphetamine at low unit doses than do IC rats (Bardo et al., 2001; Green et al., 2002). However, it is not known currently if the enrichment-induced decrease in operant responding is specific to stimulant drugs or whether it generalizes to novelty reward.
Given that environmental enrichment reduces stimulant self-administration and appears to reduce the response to novel stimuli assessed with non-operant procedures, this study sought to determine the effects of environmental enrichment on responding reinforced by a contingent novel stimulus. For this purpose, we assessed the rewarding effect of a contingent novel cue light, as this stimulus is known to serve as a reinforcer for lever pressing in rats using standard operant conditioning procedures (Barnes and Baron, 1961; Marx et al., 1955).
1. Experiment 1
In a preliminary experiment in our laboratory (Bardo and Dwoskin, 2004), EC and IC rats were allowed to earn a contingent novel visual stimulus by pressing either of two levers, i.e., both levers were active. IC rats responded more than EC rats on both levers. While these results suggest that enrichment decreased the incentive value of novelty, an alternative interpretation is that illumination of the light may have elicited an exploratory approach response (rearing) or a general increase in activity, thus resulting in increased contact with the levers. One way to rule out this latter interpretation is to use a discrimination procedure in which responding on only one lever (active) is followed by light and responding on the other lever (inactive) has no programmed consequence. If the contingent light is controlling responding, then responding should only increase to the active lever. Such a discrimination procedure has been used previously to demonstrate that environmental enrichment decreases the reinforcing effect of amphetamine (Bardo et al., 2001; Green et al., 2002). Therefore, the current experiment used a two-lever discrimination procedure to assess the reinforcing effect of novel light reward in EC and IC rats.
1.1. Methods
1.1.1. Animals
Male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN, USA) arrived in the laboratory at 21 days of age. Rats had ad libitum access to food and water throughout the experiment. The colony room was maintained at 24 °C and 45% humidity, with lights on 07:00–19:00 h. Procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and were in compliance with NIH’s Guide for the Care and Use of Laboratory Rats (1996).
1.1.2. Environmental conditions
Upon arrival, rats were assigned randomly to EC or IC home cage rearing for the duration of the study using methods described previously (Green et al., 2002; Zhu et al., 2004). EC rats were housed in a large metal cage (60 cm × 120 cm × 45 cm) with cohorts (8–10 per cage). The environment contained novel hard plastic objects (i.e., commercially available children’s toys, PVC pipe, plastic containers, etc.; 14 objects per cage). Rats were removed briefly each day so that seven of the objects could be replaced with new objects; the remaining objects were rearranged into a novel configuration. IC rats were housed individually in a stainless steel hanging cage with wire mesh floor and front panel (17 cm × 24 cm × 20 cm), and solid sides, back and top. IC rats were not handled during this rearing period (21–51 days of age).
1.1.3. Apparatus
Operant conditioning chambers (ENV-001, Med Associates St. Albans, VT) were enclosed in sound attenuating compartments and were operated by a computer interface. On the front panel of each operant conditioning chamber was a 5 cm × 4.2 cm opening that allowed access to a recessed food tray. Two metal response levers were located on either side of the food tray 7.3 cm above a metal-grid floor. A 28 V, 3-cm diameter, white cue light was centered 6 cm above each response lever.
1.1.4. Procedure
At 52 days of age, EC (n = 6) and IC (n = 6) rats were placed in the operant conditioning chamber for 60-min daily sessions. Baseline responding was measured for six sessions in which pressing on either lever in the operant chamber resulted in no programmed consequence. Following these baseline sessions, four sessions occurred in which responding on the active lever resulted in illumination of one of the cue lights, randomized between the left and right light for each response. Responding on the other lever (inactive) had no programmed consequence. To enhance the novelty of the light stimulus, the duration of illumination of each light varied between 2 and 8 s and the flash rate of each light varied between 0.2 and 0.75 s. These sessions were followed by four extinction sessions with no programmed consequence for pressing either lever.
1.1.5. Data analysis
Data for active and inactive lever responses were analyzed separately using mixed-factorial analysis of variance (ANOVA) procedures with Session as the within subjects factor and Group (EC versus IC) as the between subjects factor. The alpha level was set to .05 for all ANOVAs. Where appropriate, EC and IC groups were compared using Bonferroni corrected (p < .01) simple effects. Dunnett’s test was used to compare responding within groups across sessions.
1.2. Results
1.2.1. Baseline responding
During the baseline phase, when pressing on either lever had no programmed consequence, IC rats showed greater responding on both levers compared to EC rats (Fig. 1, left panels). ANOVA on the baseline data revealed a significant main effect of Group, F(1,10) = 10.15, p < .01. Across the six baseline sessions, responding was distributed similarly between the left and right levers for both EC and IC rats; the mean (±S.E.M.) number of presses on the left and right levers were 3.28 ± 0.95 and 2.86 ± 0.46 for EC rats and 18.36 ± 6.71 and 18.75 ± 4.19 for IC rats. Thus, since there was no lever position bias in either group during the baseline phase, the results presented in Fig. 1 on Sessions 1–6 represent the average responding on each lever regardless of position.
Fig. 1.
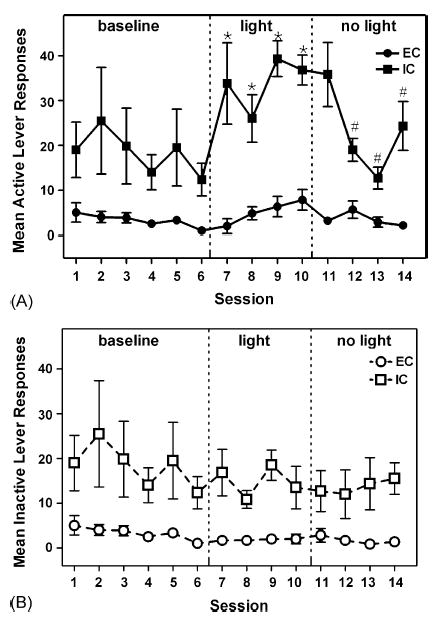
Number of active (A) and inactive (B) lever responses for EC and IC rats during the six baseline sessions (left panels), four light sessions (middle panels), and four no light sessions (right panels) in Experiment 1. Data are expressed as mean ± S.E.M. lever presses for each session. Asterisk (*) denotes significant within-subject difference compared to Session 6 and number sign (#) denotes significant within-subject difference compared to Session 10, p < .05. Note that baseline responses plotted for Sessions 1–6 (left panels) represent the average on the right or left lever alone, since neither lever was designated as active or inactive during this initial phase of the experiment.
1.2.2. Novel light stimulus available
When the contingent light was introduced on Sessions 7–10, IC rats immediately increased and maintained their responding on the active lever, whereas EC rats showed only a small gradual increase in responding on the active lever across sessions; no change was observed in either group on the inactive lever (Fig. 1, middle panels). ANOVA on data from Sessions 7 to 10 revealed a significant main effect of Group on the active lever, F(1,10) = 37.02, p < .001, and the inactive lever, F(1,10) = 13.49, p < .01, with IC rats pressing more than EC rats in each case. More important, a Dunnett’s test comparing responding during the last baseline session (Session 6) to responding during each light session (Sessions 7–10) revealed that IC rats increased responding on the active lever, but not on the inactive lever, across all four sessions. In contrast, EC rats did not significantly increase responding on either lever during any of the light sessions. In an attempt to account for the differences in baseline responding, the proportional rate of responding on the active lever was also calculated for both EC and IC rats. Similar to the analysis using raw data, when the novel light stimulus was available, IC rats displayed a significantly greater proportional increase in responding than EC rats, F(1,12) = 5.90, p < .05.
1.2.3. Novel light stimulus removed
When the contingent light was removed on Sessions 11–14, IC rats decreased their responding on the formerly active lever across sessions, whereas EC rats continued to show a low steady rate of responding (Fig. 1A, right panel). ANOVA on data from the active lever on Sessions 11–14 revealed significant main effects of Session, F(3,30) = 6.75, p < .01, and Group, F(1,10) = 26.04, p < .001, and a significant interaction between Session and Group, F(3,30) = 7.51, p < .01. ANOVA on data from the inactive lever on Sessions 11–14 also revealed a significant main effect of Group, F(1,10) = 6.53, p < .05. A Dunnett’s test comparing active lever responding during the last cue light session (Session 10) to responding during each session when the cue light was no longer available (Sessions 11–14) revealed that IC rats significantly decreased responding on Sessions 12–14. There was no significant change in responding for EC rats. Examination of the proportional rate of responding on the active lever also revealed that IC rats significantly decreased their responding on Sessions 12 and 13, while there was no significant change in EC rats. Thus, introduction of the contingent light increased responding on the active lever in IC rats only, and this increase was extinguished by removal of the light.
2. Experiment 2
The purpose of the Experiment 2 was two-fold. First, in order to equate baseline responding in EC and IC rats, a procedure was used in which rats were first pretrained to press a lever for sucrose reward prior to introduction of the contingent light. Second, in order to determine if the effect of enrichment reflected the presence of social cohorts or novel objects, another group of rats raised in a social condition (SC) without novel objects was included in the experimental design.
2.1. Methods
2.1.1. Animals
EC and IC rats were raised as described previously in Experiment 1. SC rats were housed in pairs in a clear polycarbonate tub (20 cm × 43 cm × 20 cm) with a wire rack top and handled only during the weekly cage change. This housing condition was chosen because it conforms to the typical housing condition set by NIH guidelines (1996).
2.1.2. Apparatus
The apparatus was the same as used in Experiment 1.
2.1.3. Procedure
At 52 days of age, EC (n = 20), IC (n = 20), and SC (n = 20) rats were deprived to 85% of their ad libitum weights by restricting their intake of rat chow to 5–10 g per day for 7 days. EC and SC rats were separated from cohorts during the feeding period (60 min/day). Subsequently, rats were trained to eat 20 sucrose pellets (Noyes, 45 mg) delivered to the food tray in the operant conditioning chamber. On the following day, they were trained on a continuous reinforcement schedule of food reinforcement for a 15-min period and were required to earn 100 reinforcers with only the active lever available. The rats were trained for an additional 3 days on sucrose reinforcement. During these three pretraining sessions, both levers were available; one lever delivered the sucrose pellet (active lever) and the second lever had no programmed consequence (inactive lever).
Following pretraining, rats were given ad libitum access to food to regain their free-feed body weight and were maintained on ad libitum food access for the remainder of the experiment. EC, IC, and SC rats were then divided randomly into a Light group (EC = 12, IC = 12, SC = 12) and a Control group (EC = 8, IC = 8, SC = 8). For the Light group, four sessions occurred in which responding on the active lever (same as during pretrain-ing) resulted in illumination of one of the cue lights, randomized between the left and right light for each response. Responding on the other lever (inactive) had no programmed consequence. The duration and flash rate of the light varied as described in Experiment 1. These sessions were followed by four extinction sessions with no programmed consequence for pressing either lever. For the Control group, pressing on the active lever (same as during pretraining) had no programmed consequence during Sessions 1–8. Following Session 8, rats in the Control group were given an additional four sessions in which pressing the active lever resulted in contingent light.
2.1.4. Data analysis
Data for active and inactive lever responses were analyzed separately using mixed-factorial analysis of variance procedures, with Session as the within subjects factor and Group (EC versus SC versus IC) and Order (Light versus Control) as the between subjects factors. The alpha level was set to .05 for all ANOVAs. Where appropriate, EC, SC and IC groups were compared using Bonferroni corrected (p < .01) simple effects. Dunnett’s test was used to compare responding within groups across sessions.
2.2. Results
2.2.1. Sucrose pretraining
Overall, EC, SC, and IC rats did not differ in active lever responding during initial sucrose pretraining (mean ± S.E.M. for EC = 114.87 ± 8.39; IC = 125.28 ± 3.18; SC = 108.10 ± 6.48). ANOVA revealed only a main effect of Session, F(2,114) = 12.70, p < .001, with responding increasing across sessions. An ANOVA performed on responses on the inactive lever revealed a main effect of Session, F(2,114) = 53.43, p < .001, and Group, F(2,57) = 4.13, p < .05, as well as a significant interaction between Session and Group, F(4,114) = 3.56, p < .01. Despite the significant interaction, responding among groups did not differ significantly on any session (mean ± S.E.M. for EC = 5.82 ± 1.30; IC = 5.75 ± 0.72; SC = 10.03 ± 1.47).
2.2.2. Effects of the novel light stimulus on active lever responding
When the light contingency was introduced on Sessions 1–4 for the Light group, SC rats responded for the light, whereas EC and IC rats did not (Fig. 2, left panels). ANOVA performed on the active lever revealed a significant main effect of Session, F(3,162) = 101.43, p < .001, Group, F(2,54) = 25.96, p < .001, and Order, F(1,54) = 6.99, p < .05, as well as significant interactions between Session and Group, F(6,162) = 3.12, p < .01, and between Session and Order, F(3,162) = 10.48, p < .001. SC rats in the Light group responded significantly more than SC rats in the Control group during Session 1, F(1,18) = 13.92, p < .01, and Session 3, F(1,18) = 10.87, p < .01.
Fig. 2.
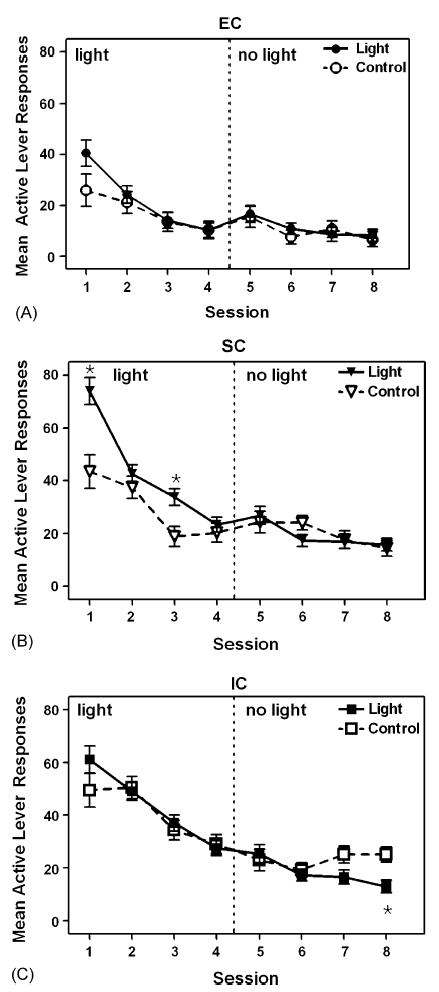
Number of active lever responses for EC (A), SC (B), and IC (C) rats in the Light and Control groups in Experiment 2. In the light phase (Sessions 1–4), the Light group received contingent light; in the no light phase (Sessions 5–8), neither group received contingent light. Data are expressed as mean ± S.E.M. lever presses for each session. Asterisk (*) denotes significant difference (p < .01) from the Control group.
When the light contingency was omitted on Sessions 5–8, there was general decrease in responding on the active lever across sessions; however, there was no notable change in responding attributable to the removal of the light contingency in any group (Fig. 2, right panels). ANOVA revealed significant main effects of Session, F(3,162) = 15.62, p < .001, and Group, F(2,54) = 10.78, p < .001, as well as a significant three-way interaction involving Session, Group, and Order, F(6,162) = 2.37, p < .05. IC rats in the Light group responded significantly less than IC rats in the Control group on Session 8, F(1,18) = 5.57, p < .01. A Dunnett’s test comparing responding on the active lever on Session 4 to responding on Sessions 5–8 revealed no significant differences in EC, SC, or IC rats. Thus, responding on the active lever in the Light and Control groups given sucrose-reinforced pretraining provided little evidence that the contingent light engendered reliable responding.
2.2.3. Effects of the novel light stimulus on inactive lever responding
Responding on the inactive lever across Sessions 1–8 also revealed no reliable differences between the Light and Control groups, regardless of rearing condition (Fig. 3). ANOVA performed on the data from Sessions 1 to 4 revealed main effects of Session, F(3,162) = 11.35, p < .001, and Group, F(2,54) = 44.47, p < .001, as well as an interaction between Session and Group, F(6,162) = 2.85, p < .05. A similar ANOVA on Sessions 5–8 revealed a significant main effect of Group, F(2,54) = 25.59, p < .001. Across sessions, the response rate was lower in EC rats than in SC and IC rats.
Fig. 3.
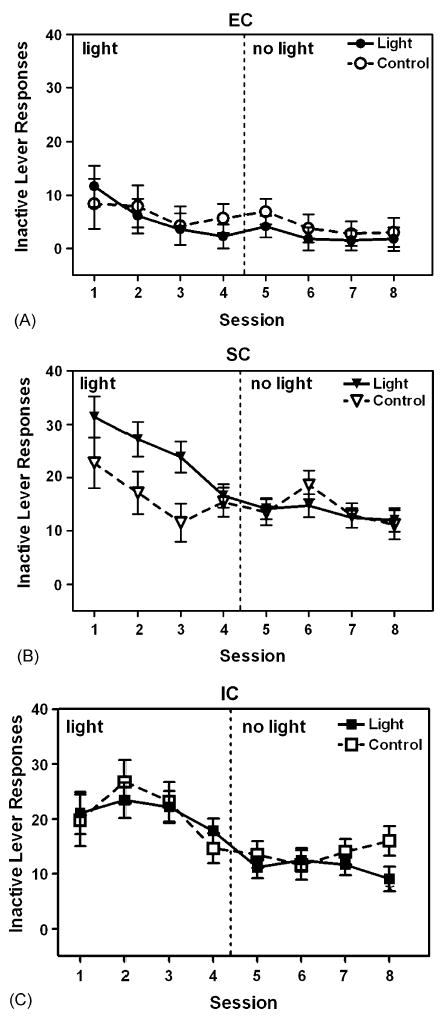
Number of inactive lever responses for EC (A), SC (B), and IC (C) rats in the Light and Control groups in Experiment 2. In the light phase (Sessions 1–4), the Light group received contingent light; in the no light phase (Sessions 5–8), neither group received contingent light. Data are expressed as mean ± S.E.M. lever presses for each session.
2.2.4. Effects of the novel light stimulus on Control group responding
In the final phase of the experiment, when a contingent light was introduced for the Control group on Sessions 9–12, there was a clear increase in responding on the active lever, with the effect being most marked in the IC rats and least marked in the EC rats (Fig. 4). ANOVA on data from the active lever revealed significant main effects of Session, F(3,63) = 47.41, p < .001, and Group, F(2,21) = 23.53, p < .001, as well as a significant interaction between Session and Group, F(6,63) = 5.30, p < .001. On Session 9, when the contingent light was first introduced, IC rats responded significantly more on the active lever than EC and SC rats, while SC rats responded significantly more than EC rats. Dunnett’s tests comparing responding during the last session without light (Session 8) to responding during each light session (Sessions 9–12) revealed that both EC and IC rats significantly increased their responding on the active lever during Sessions 9 and 10, while SC rats significantly increased their responding during Sessions 9–11. In contrast, ANOVA on data from the inactive lever revealed no significant effects (see Fig. 4), indicating that the effect of enrichment was specific to the active lever. The proportional rate of responding on the active lever was also calculated for EC, IC, and SC rats in the Control group. During Session 9, the proportional rate of responding in IC rats was significantly more than EC and SC rats, while the proportional rate of responding in SC rats was significantly more than EC rats. During Session 10, the proportional rate of responding in IC rats was significantly more than EC rats. There were no differences in the proportional rates of responding between the groups during Sessions 11 and 12. Thus, although all groups responded for the contingent light, the strength of responding was greatest in IC rats and least in EC rats.
Fig. 4.
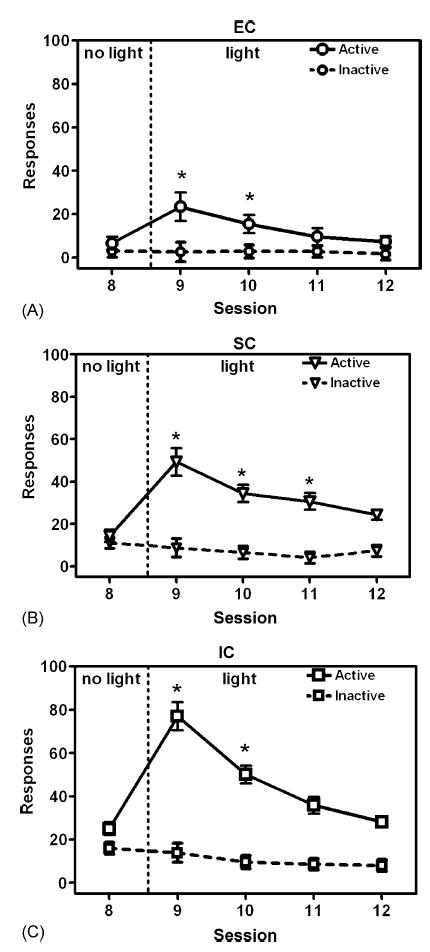
Number of active and inactive lever responses for EC (A), SC (B), and IC (C) rats in the Control group during the last session with no light (Session 8) and the four sessions with light (Sessions 9–12) in Experiment 2. Data are expressed as mean ± S.E.M. lever presses for each session. Asterisk (*) denotes a significant within-subject difference (p < .05) compared to Session 8.
3. General discussion
The current results suggest that differential rearing alters the incentive salience of visual novelty. In Experiment 1, baseline lever pressing (non-reinforced) was initially lower in EC rats than IC rats, and when a contingent light was introduced, there was an immediate and prolonged increase in responding in IC rats, but not EC rats. When the contingent light was omitted, only IC rats continued to show increased responding on the previously reinforced lever. In Experiment 2, a sucrose-reinforced pretraining procedure was used to increase the baseline response rate to similar levels in EC and IC rats. While this procedure equated baseline responding, introduction of the contingent light after pretraining failed to control responding in either group, suggesting that prior lever press training for sucrose reward negated the relative impact of the novel light. Nonetheless, after eight sessions of extinction of the sucrose-reinforced lever press, introduction of the contingent light was again able to control responding, with IC rats showing the greatest increase and EC rats showing the least increase in responding; performance of SC rats was intermediate between the other two groups. Taken together, these findings suggest that EC rats are less responsive to novelty reward.
The observation that a light stimulus can serve as a reinforcer has been demonstrated previously. While early reports suggested that light stimulation is an aversive stimulus for the albino rat (Keller, 1941; Flynn and Jermone, 1952; Kaplan, 1952), Marx et al. (1955) demonstrated that rats tested under food restriction will increase responding for a 1-s light stimulus, suggesting that light itself serves as a reinforcer. Further, under a food-restricted condition, it has been observed that IC rats increase responding, while EC rats decrease responding, when a 1-s high intensity light is paired with the sound of the lever and pellet dispenser (Rose et al., 1986). The current study extends this original work by showing that rats also respond for a novel light stimulus (varying duration and flash rate) at free-feed body weight, indicating that the motivation to respond for contingent light does not simply result from food deprivation. In addition, the current study used a discrimination procedure to rule out direct effects of the light and included a SC group for comparison.
During the baseline phase of Experiment 1, EC rats emitted significantly fewer non-reinforced responses than did IC rats. This finding is consistent with previous observations that EC rats display less activity in an inescapable novel environment (Lore and Levowitz, 1966; Fuller, 1967; Bowling et al., 1993; Smith et al., 1997; Green et al., 2003), respond less for a weak auditory stimulus in the absence of consummatory reward (Rose et al., 1986), and have lower response rates on a DRL-20 s operant task (Ough et al., 1972). Since EC rats tend to have reduced locomotor activity compared to IC rats, it could be argued that the effects observed in these studies are simply due to the lower baseline activity of EC rats. However, this conclusion is unlikely because the difference observed between EC and IC rats was observed regardless whether the results were expressed as absolute number of responses or a proportional change in the number of responses.
It is possible that the current findings may be interpreted to reflect an effect of impoverishment rather than enrichment. The enriched and impoverished conditions differ in the amount of handling, physical activity, and social interactions, size of the home cage, complexity of visual and tactile experiences, and presence of novel objects (Renner and Rosenzweig, 1987). In an attempt to examine this issue more thoroughly, we also compared the responding of EC rats to a group of SC rats. EC rats responded less for a reinforcer than SC rats (Experiment 2), suggesting that enrichment decreases responding for novelty. While the SC group in this study does not control for all the differences between the IC and EC animals, it does indicate that at least a portion of the difference between EC and IC rats is due to exposure to the novel objects, rather than simply social cohorts.
In conclusion, the current results show that, similar to the results obtained with amphetamine self-administration (Bardo et al., 2001; Green et al., 2002), environmental enrichment decreases the incentive salience of a novel non-drug reinforcer. Since novelty and drugs of abuse are thought to share similar neural substrates (Feenstra and Botterblom, 1996; Rebec et al., 1997; Beaufour et al., 2001), it is possible that repeated exposure to enriching novel stimulation during development may activate these substrates repeatedly, thus reducing the relative reinforcing strength of subsequent reinforcers. Recent neurochemical work from our laboratory suggests that this environment-induced decrease in incentive salience may involve, at least in part, a change in dopamine transporter function in the medial prefrontal cortex (Zhu et al., 2004, 2005).
Acknowledgments
Research was funded by USPHS Grant R01 DA12964. MEC supported by USPHS Grant F32 DA16013 and T.A.G. supported by USPHS Grant F31 DA06093. The authors would like to thank Kathryn Bylica, Laura Fenton, and Brenna Shortridge for their assistance with this project, and Dr. Rick Bevins for some helpful discussion regarding the work.
References
- Barnes GW, Baron A. The effect pf sensory reinforcement on extinction behavior. Journal of Comparative and Physiological Psychology. 1961;54:461–465. doi: 10.1037/h0040590. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Dwoskin LP. Biological connection between novelty-seeking and drug-seeking motivational systems. In: Bevins RA, Bardo MT, editors. Motivational Factors in the Etiology of Drug Abuse. Vol. 50. University of Nebraska Press; Lincoln: 2004. pp. 127–158. [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behavioral Brain Research. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Beaufour CC, Le Bihan C, Hamon M, Thiebot M. Extracellular dopamine in the rat prefrontal cortex during reward-, punishment- and novelty-associated behaviour. Effects of diazepam Pharmacol Biochem Behav. 2001;69:133–142. doi: 10.1016/s0091-3057(01)00492-0. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Schwegler H, van Abeelen JHF. Behavioral responses to novelty and structural variation of the hippocampus in mice. I Quantitative-genetic analysis of behavior in the open-field. Behav Brain Res. 1989;32:75–80. doi: 10.1016/s0166-4328(89)80074-9. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Res. 1996;742:17–24. doi: 10.1016/s0006-8993(96)00945-6. [DOI] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Decreased locomotor and investigatory exploration after denervation of catecholamine terminal fields in the forebrain of rats. J Comp Physiol Psychol. 1979;93:34–65. doi: 10.1037/h0077587. [DOI] [PubMed] [Google Scholar]
- Flynn JP, Jermone EA. Learning in an automatic multiple-choice box with light as incentive. J Comp Physiol Psychol. 1952;45:336–340. doi: 10.1037/h0054587. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Experiential deprivation and later behavior. Science. 1967;158:1645–1652. doi: 10.1126/science.158.3809.1645. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 2003;170:235–241. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources. National Research Council, National Academy Press; Washington, DC: 1996. in press. [Google Scholar]
- Kaplan M. The effects of noxious stimulus intensity and duration suring intermittent reinforcement of escape behavior. J Comp Physiol Psychol. 1952;45:538–549. doi: 10.1037/h0055989. [DOI] [PubMed] [Google Scholar]
- Keller FS. Light aversion in the white rat. Psychol Rec. 1941;4:235–250. [Google Scholar]
- Lore RK, Levowitz A. Differential rearing and free versus forced exploration. Psychon Sci. 1966;5:421–422. [Google Scholar]
- Marx MH, Henderson RL, Roberts CL. Positive reinforcement of the bar-pressing response by a light stimulus following dark operant pretests with no after effect. J Comp Physiol Psychol. 1955;48:73–76. doi: 10.1037/h0045062. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen M. Neuropharmacological evidence to suggest that the nucleus accumbens and subpallidal region contribute to exploratory locomotion. Behav Neural Biol. 1984;42:52–60. doi: 10.1016/s0163-1047(84)90424-2. [DOI] [PubMed] [Google Scholar]
- Oliverio A, Messeri P. An analysis of single-gene effects on avoidance, maze, wheel running, and exploratory behavior in the mouse. Behav Biol. 1973;8:771–783. doi: 10.1016/s0091-6773(73)80120-8. [DOI] [PubMed] [Google Scholar]
- Ough BR, Beatty WA, Khalili J. Effects of isolated and enriched rearing on response inhibition. Psychon Sci. 1972;27:293–294. [Google Scholar]
- Peeler DF, Nowakowski RS. Genetic factors and the measurement of exploratory activity. Behav Neural Biol. 1987;48:90–103. doi: 10.1016/s0163-1047(87)90619-4. [DOI] [PubMed] [Google Scholar]
- Rebec Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- Renner MJ, Rosenzweig MR. Object interactions in juvenile rats (Rattus norvegicus): effects of different experimental histories. J Comp Psychol. 1986;100:229–236. [Google Scholar]
- Renner MJ, Rosenzweig MR. Enriched and Impoverished Environments: Effects on Brain and Behavior. Springer-Verlag; New York: 1987. [Google Scholar]
- Rose FD, Love S, Dell PA. Differential reinforcement effects in rats reared in enriched and impoverished environments. Physiol Behav. 1986;36:1139–1145. doi: 10.1016/0031-9384(86)90491-9. [DOI] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioral effects of cocaine and d-amphetamine. Psychopharmacology. 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Widman DR, Rosellini RA. Restricted daily exposure to environmental enrichment increases the diversity of exploration. Physiol Behav. 1990;47:57–62. doi: 10.1016/0031-9384(90)90042-3. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green TA, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann A, Stauffacher M, Langhans W, Wurbel H. Enrichment-dependent differences in novelty exploration in rats can be explained by habituation. Behav Brain Res. 2001;121:11–20. doi: 10.1016/s0166-4328(00)00377-6. [DOI] [PubMed] [Google Scholar]


