Abstract
1. Sodium amylobarbitone (3·54 mg/kg) was given by intravenous injection to seven healthy men and nine healthy women who were not receiving other drugs. Serum amylobarbitone and urine hydroxyamylobarbitone concentrations were measured by gas-liquid chromatography. There was no significant difference between the groups either in the serum amylobarbitone concentration/time curves or in the urinary excretion of hydroxyamylobarbitone.
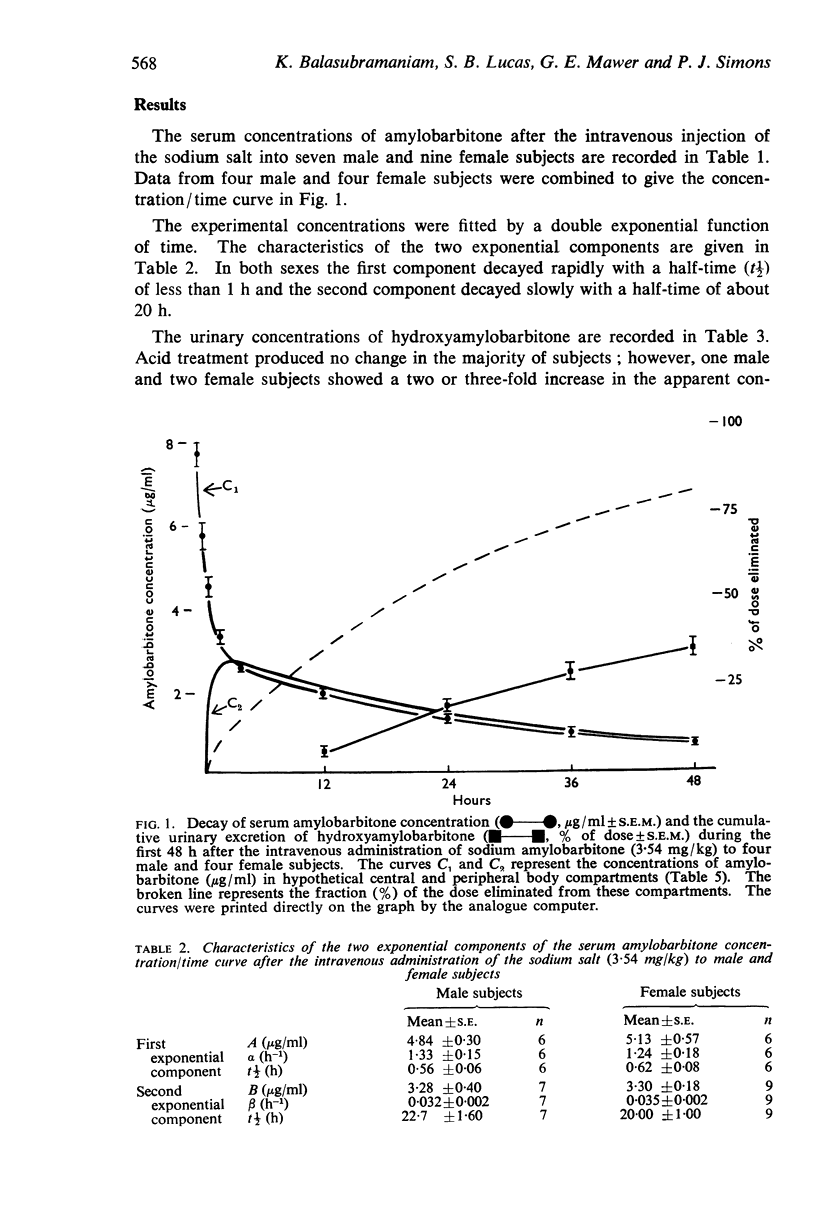
2. The serum amylobarbitone concentration decayed over 48 h as a double exponential function of time; the first exponential component had a mean half-time of 0·6 h (males 0·56 ± 0·06 h, females 0·62 ± 0·08 h, ± S.E.) and the second exponential component had a mean half time of 21 h (males 22·7 ± 1·6 h, females 20·0 ± 1·0 h, ± S.E.).
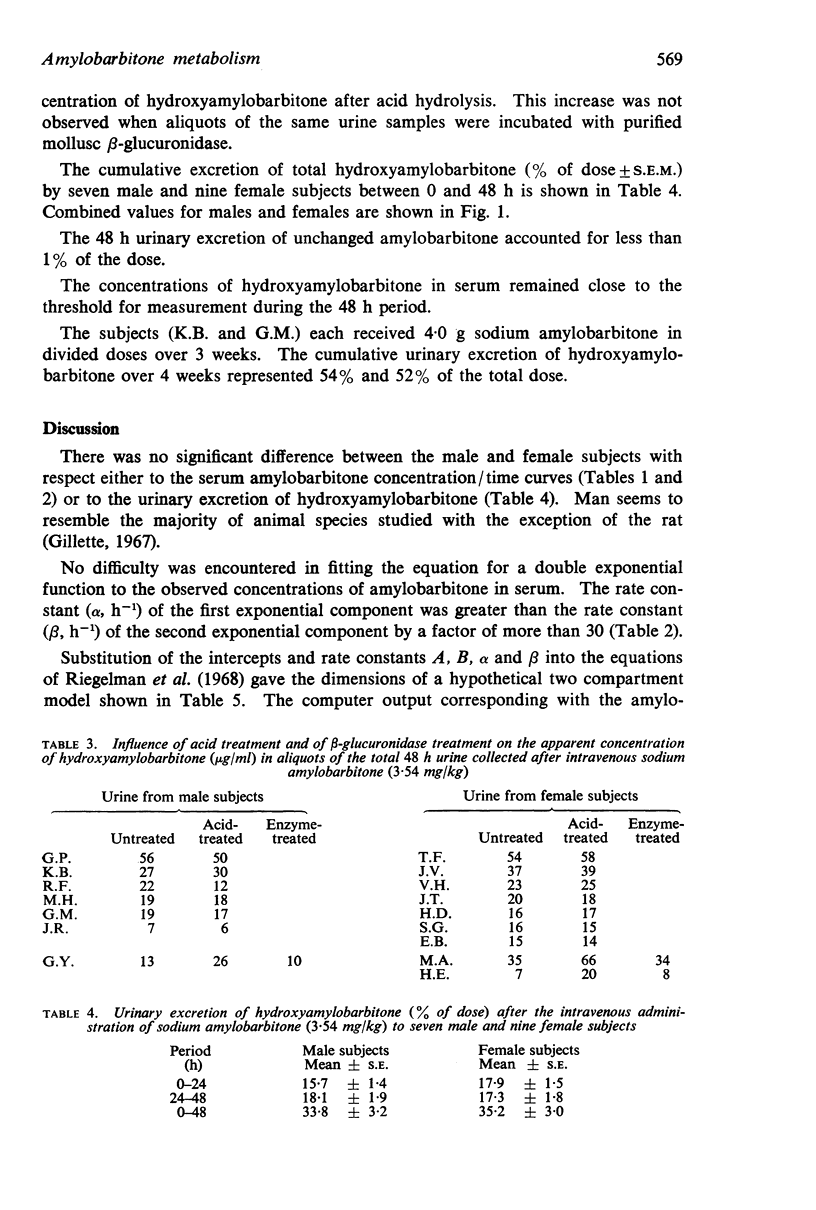
3. The urinary excretion of hydroxyamylobarbitone over 48 h accounted for 34% of the dose (males 33·8 ± 3·2%, females 35·2 ± 3·0%, ± S.E.). One male and two female subjects excreted hydroxyamylobarbitone partly as a conjugate which was readily hydrolysed in acid.
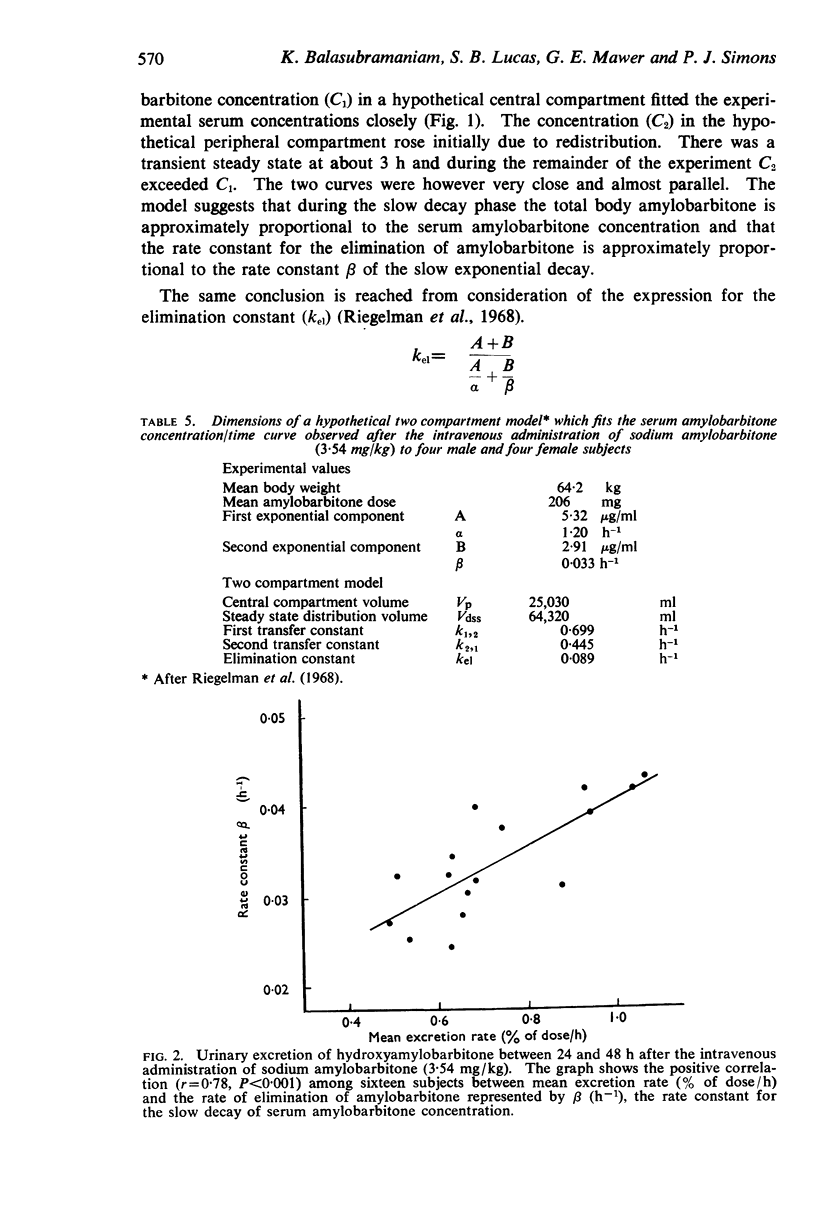
4. An elimination constant (kel) derived from the serum concentration/time curve by the application of a two compartment model was approximately proportional to β (h-1), the rate constant of the second exponential component. There was a positive correlation (r=0·78, P<0·001) between β and the mean rate of urinary excretion of hydroxyamylobarbitone during the 24 to 48 h period.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER T. C. The metabolic hydroxylation of phenobarbital. J Pharmacol Exp Ther. 1956 Mar;116(3):326–336. [PubMed] [Google Scholar]
- Balasubramaniam K., Mawer G. E., Rodgers E. M. The estimation of amylobarbitone and hydroxyamylobarbitone in serum by gas liquid chromatography. Br J Pharmacol. 1969 Oct;37(2):546P–547P. [PMC free article] [PubMed] [Google Scholar]
- Bush M. T., Sanders E. Metabolic fate of drugs: barbiturates and closely related compounds. Annu Rev Pharmacol. 1967;7:57–76. doi: 10.1146/annurev.pa.07.040167.000421. [DOI] [PubMed] [Google Scholar]
- Kamm J. J., Van Loon E. J. Amobarbital metabolism in man. A gas chromatographic method for the estimation of hydroxyamobarbital in human urine. Clin Chem. 1966 Nov;12(11):789–796. [PubMed] [Google Scholar]
- MAYNERT E. W. Ethyl (3-hydroxyisoamyl) barbituric acid as the principal metabolite of amytal. J Biol Chem. 1952 Mar;195(1):397–402. [PubMed] [Google Scholar]
- Mawer G. E., Lee H. A. Value of forced diuresis in acute barbiturate poisoning. Br Med J. 1968 Jun 29;2(5608):790–793. doi: 10.1136/bmj.2.5608.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynert E. W. The alcoholic metabolites of pentobarbital and amobarbital in man. J Pharmacol Exp Ther. 1965 Oct;150(1):118–121. [PubMed] [Google Scholar]
- RAVENTOS J. The distribution in the body and metabolic fate of barbiturates. J Pharm Pharmacol. 1954 Apr;6(4):217–235. [PubMed] [Google Scholar]
- RICHARDS R. K., TAYLOR J. D. Some factors influencing distribution, metabolism and action of barbiturates; a review. Anesthesiology. 1956 May;17(3):414–458. doi: 10.1097/00000542-195605000-00005. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Loo J. C., Rowland M. Shortcomings in pharmacokinetic analysis by conceiving the body to exhibit properties of a single compartment. J Pharm Sci. 1968 Jan;57(1):117–123. doi: 10.1002/jps.2600570123. [DOI] [PubMed] [Google Scholar]
- Schenkman J. B., Frey I., Remmer H., Estabrook R. W. Sex differences in drug metabolism by rat liver microsomes. Mol Pharmacol. 1967 Nov;3(6):516–525. [PubMed] [Google Scholar]


