Abstract
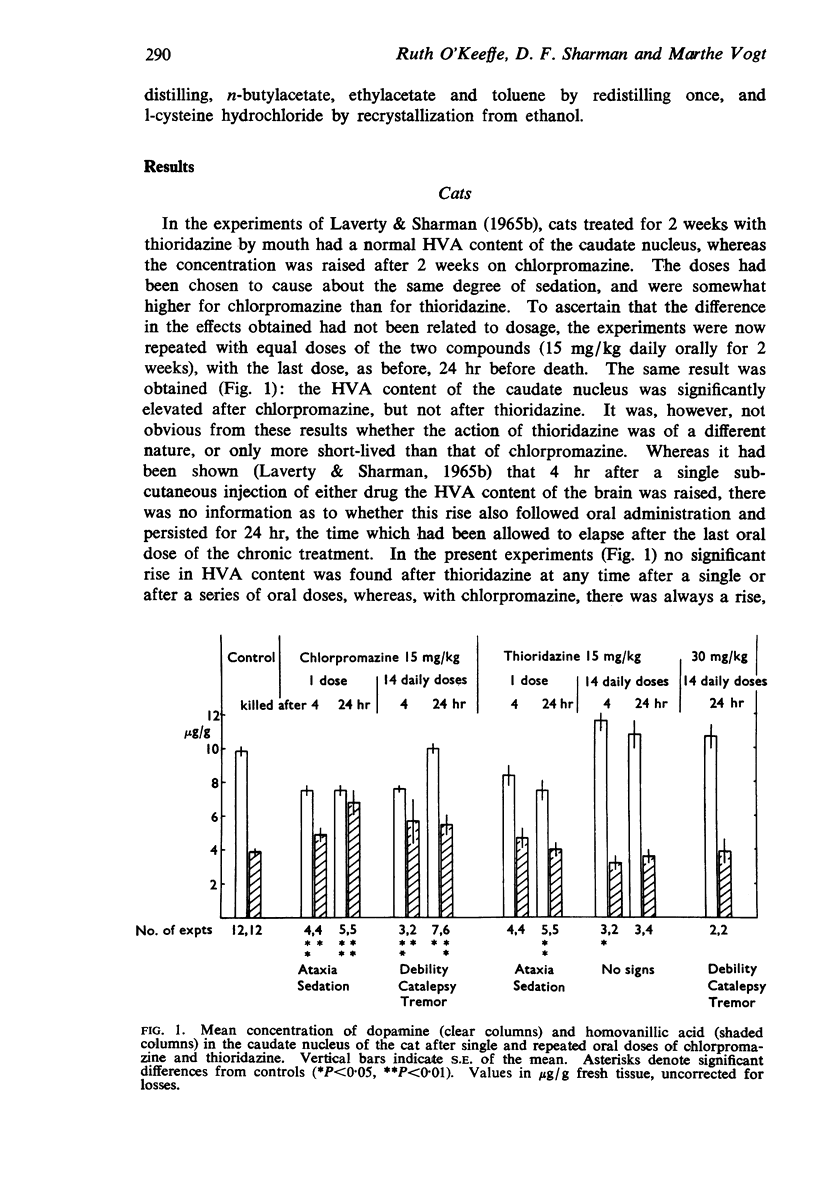
1. Chlorpromazine 15 mg/kg, given daily to cats for 2 weeks, produced a rise in homovanillic acid (HVA) content of the caudate nucleus, whereas the same dose of thioridazine lacked this effect. Of these two drugs, only chlorpromazine causes a high incidence of drug-induced Parkinsonism in man.
2. In the mouse, chlorpromazine, thioridazine and haloperidol increased striatal concentrations of HVA and accelerated the disappearance of dopamine (DA) after inhibition of catecholamine synthesis with α-methyltyrosine. Low doses of the three compounds increased, whereas high doses reduced, the concentration of DA in the striatum. In their effects on the DA metabolism of the mouse, chlorpromazine and thioridazine had the same potency, but haloperidol was between 10 and 100 times more active than the other two drugs. In producing hypothermia and sedation, the three compounds were equiactive.
3. Oxypertine, another drug apt to produce Parkinsonism in man, caused a severe reduction in striatal DA and hypothalamic noradrenaline (NA). Though the clinical signs produced in the mouse were indistinguishable from those seen after the same dose of chlorpromazine, the biochemical changes in the brain were thus quite different.
4. Though all the drugs used caused temporary motor disabilities in animals, these bore no resemblance to human Parkinsonism, even when treatment was continued for 7 weeks or more as it was in cats and monkeys. The latter were treated with chlorpromazine 7·5 mg/kg daily, a dose chosen to avoid loss of weight and which may have been too small to produce toxic side-effects. It caused no changes in striatal DA turnover.
5. Even at the high dose of 50 mg/kg, phenoxybenzamine did not increase DA turnover in mouse brain, but it sedated the mice as did the tranquillizers.
6. Atropine sulphate, 25 mg/kg, reduced the HVA content of mouse striatum and partially antagonized the rise in HVA produced by phenothiazines. The effect was surmountable. Possible modes of action of atropine are discussed.
7. At present we know of two types of biochemical changes which may occur in the brain of animals after treatment with drugs apt to cause Parkinsonism in man: a loss of cerebral catecholamines, as seen after reserpine or oxypertine, or an increase in turnover of DA as after phenothiazines and butyrophenones.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson J., Roos B. E., Steg G. Reciprocal effects on alpha- and gamma-motoneurones of drugs influencing monoaminergic and cholinergic transmission. Acta Physiol Scand. 1966 Jul-Aug;67(3):398–404. doi: 10.1111/j.1748-1716.1966.tb03326.x. [DOI] [PubMed] [Google Scholar]
- BUTLER W. M., Jr, MORAN N. C. The pharmacological properties of chlorpromazine sulfoxide, a major metabolite of chlorpromazine; a comparison with chlorpromazine. J Pharmacol Exp Ther. 1956 Nov;118(3):328–337. [PubMed] [Google Scholar]
- Brodie B. B., Comer M. S., Costa E., Dlabac A. The role of brain serotonin in the mechanism of the central action of reserpine. J Pharmacol Exp Ther. 1966 May;152(2):340–349. [PubMed] [Google Scholar]
- CARLSSON A., LINDQVIST M. EFFECT OF CHLORPROMAZINE OR HALOPERIDOL ON FORMATION OF 3METHOXYTYRAMINE AND NORMETANEPHRINE IN MOUSE BRAIN. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- COLE J. O., CLYDE D. J. Extrapyramidal side effects and clinical response to the phenothiazines. Rev Can Biol. 1961 Jun;20:565–574. [PubMed] [Google Scholar]
- HOLLISTER L. E., OVERALL J. E., KIMBELL I., Jr, BENNETT J. L., MEYER F., CAFFEY E., Jr Oxypertine in newly admitted schizophrenics. J New Drugs. 1963 Jan-Feb;3:26–31. doi: 10.1002/j.1552-4604.1963.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Himwich W. A., Glisson S. N. Effect of haloperidol on caudate nucleus. Int J Neuropharmacol. 1967 Jul;6(4):329–332. doi: 10.1016/0028-3908(67)90023-8. [DOI] [PubMed] [Google Scholar]
- JUORIO A. V., VOGT M. THE EFFECT OF PRENYLAMINE ON THE METABOLISM OF CATECHOL AMINES AND 5-HYDROXYTRYPTAMINE IN BRAIN AND ADRENAL MEDULLA. Br J Pharmacol Chemother. 1965 Apr;24:566–573. doi: 10.1111/j.1476-5381.1965.tb01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juorio A. V., Sharman D. F., Trajkov T. The effect of drugs on the homovanillic acid content of the corpus striatum of some rodents. Br J Pharmacol Chemother. 1966 Feb;26(2):385–392. doi: 10.1111/j.1476-5381.1966.tb01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juorio A. V., Vogt M. Monoamines and their metabolites in the avian brain. J Physiol. 1967 Apr;189(3):489–518. doi: 10.1113/jphysiol.1967.sp008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAELBER W. W., JOYNT R. J. Tremor production in cats given chlorpromazine. Proc Soc Exp Biol Med. 1956 Jun;92(2):399–402. doi: 10.3181/00379727-92-22491. [DOI] [PubMed] [Google Scholar]
- LAVERTY R., SHARMAN D. F. MODIFICATION BY DRUGS OF THE METABOLISM OF 3,4-DIHYDROXYPHENYLETHYLAMINE, NORADRENALINE AND 5-HYDROXYTRYPTAMINE IN THE BRAIN. Br J Pharmacol Chemother. 1965 Jun;24:759–772. doi: 10.1111/j.1476-5381.1965.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVERTY R., SHARMAN D. F. THE ESTIMATION OF SMALL QUANTITIES OF 3,4-DIHYDROXYPHENYLETHYLAMINE IN TISSUES. Br J Pharmacol Chemother. 1965 Apr;24:538–548. doi: 10.1111/j.1476-5381.1965.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUOKA M. FUNCTION AND METABOLISM OF CATECHOLAMINES IN THE BRAIN. Jpn J Pharmacol. 1964 Jun;14:181–193. doi: 10.1254/jjp.14.181. [DOI] [PubMed] [Google Scholar]
- Maickel R. P. Diverse central effects of chlorpromazine. Int J Neuropharmacol. 1968 Jan;7(1):23–27. doi: 10.1016/0028-3908(68)90051-8. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Ishi S., Shimizu N., Imaizumi R. Effect of Win 18501-2 content of catecholamines and the number of catecholamine-containing granules in the rabbit hypothalamus. Experientia. 1965 Mar 15;21(3):121–123. doi: 10.1007/BF02141966. [DOI] [PubMed] [Google Scholar]
- Poirier L. J., Sourkes T. L., Bouvier G., Boucher R., Carabin S. Striatal amines, experimental tremor and the effect of harmaline in the monkey. Brain. 1966 Mar;89(1):37–52. doi: 10.1093/brain/89.1.37. [DOI] [PubMed] [Google Scholar]
- Portig P. J., Sharman D. F., Vogt M. Release by tubocurarine of dopamine and homovanillic acid from the superfused caudate nucleus. J Physiol. 1968 Feb;194(2):565–572. doi: 10.1113/jphysiol.1968.sp008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARMAN D. F., VOGT M. THE NORADRENALINE CONTENT OF THE CAUDATE NUCLEUS OF THE RABBIT. J Neurochem. 1965 Jan;12:62–62. doi: 10.1111/j.1471-4159.1965.tb10254.x. [DOI] [PubMed] [Google Scholar]
- SPECTOR S., MELMON K., SJOERDSMA A. Evidence for rapid turnover of norepinephrine in rat heart and brain. Proc Soc Exp Biol Med. 1962 Oct;111:79–81. doi: 10.3181/00379727-111-27710. [DOI] [PubMed] [Google Scholar]
- Sharman D. F. A discussion of the modes of action of drugs which increase the concentration of 4-hydroxy-3-methoxyphenylacetic acid (homovanillic acid) in the striatum of the mouse. Br J Pharmacol Chemother. 1967 Aug;30(3):620–626. doi: 10.1111/j.1476-5381.1967.tb02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman D. F. Changes in the metabolism of 3,4-dihydroxyphenylethylamine (dopamine) in the striatum of the mouse induced by drugs. Br J Pharmacol Chemother. 1966 Nov;28(2):153–163. doi: 10.1111/j.1476-5381.1966.tb01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillito E. E. The effect of chlorpromazine and thioridazine on the exploration of a Y-maze by rats. Br J Pharmacol Chemother. 1967 Jun;30(2):258–264. doi: 10.1111/j.1476-5381.1967.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelik P. G., Ernst A. M. Role of nigro-neostriatal dopaminergic fibers in compulsive gnawing behavior in rats. Life Sci. 1966 Aug;5(16):1485–1488. doi: 10.1016/0024-3205(66)90222-0. [DOI] [PubMed] [Google Scholar]


