Abstract
1. A synthetic oxytocin analogue, [1-N-carbamoyl-hemicystine-2-O-methyltyrosine]-oxytocin (carbamoyl-methyloxytocin), has been tested as an antagonist to the actions of oxytocin and vasopressin on the uterus, the mammary gland and blood pressure.
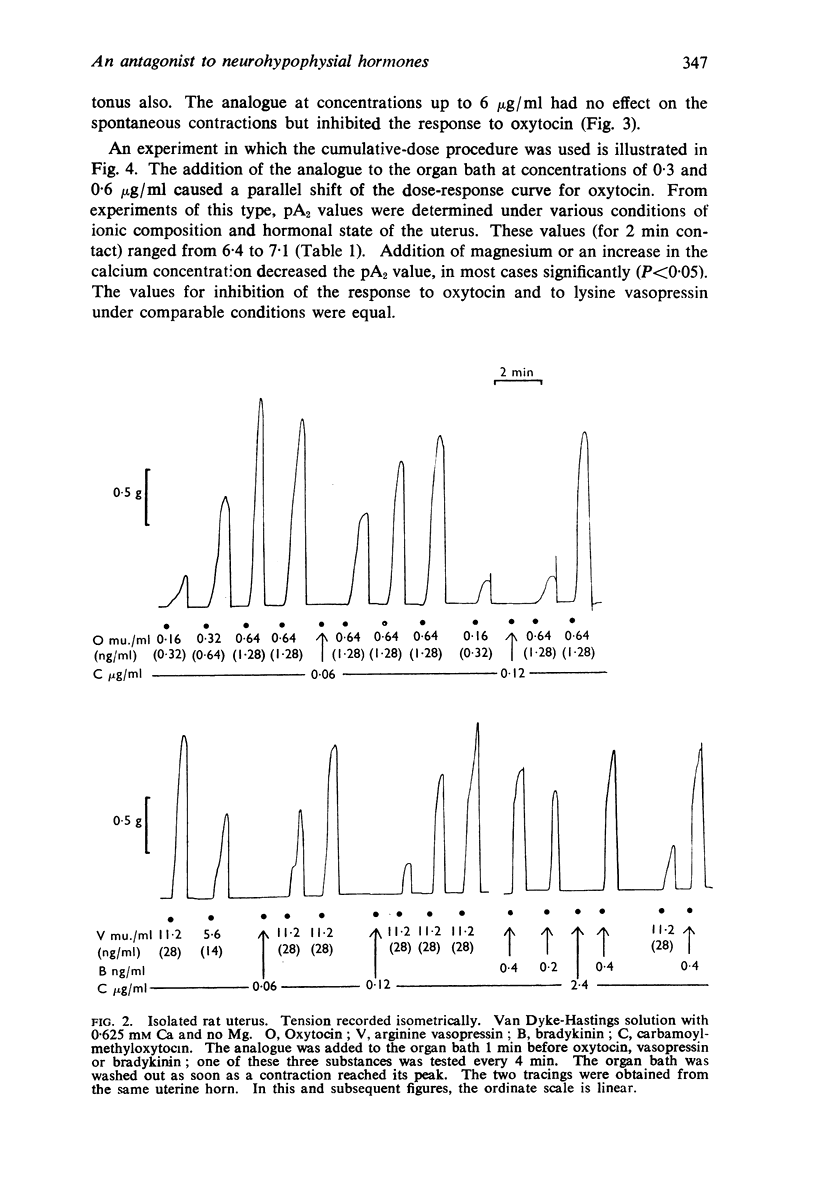
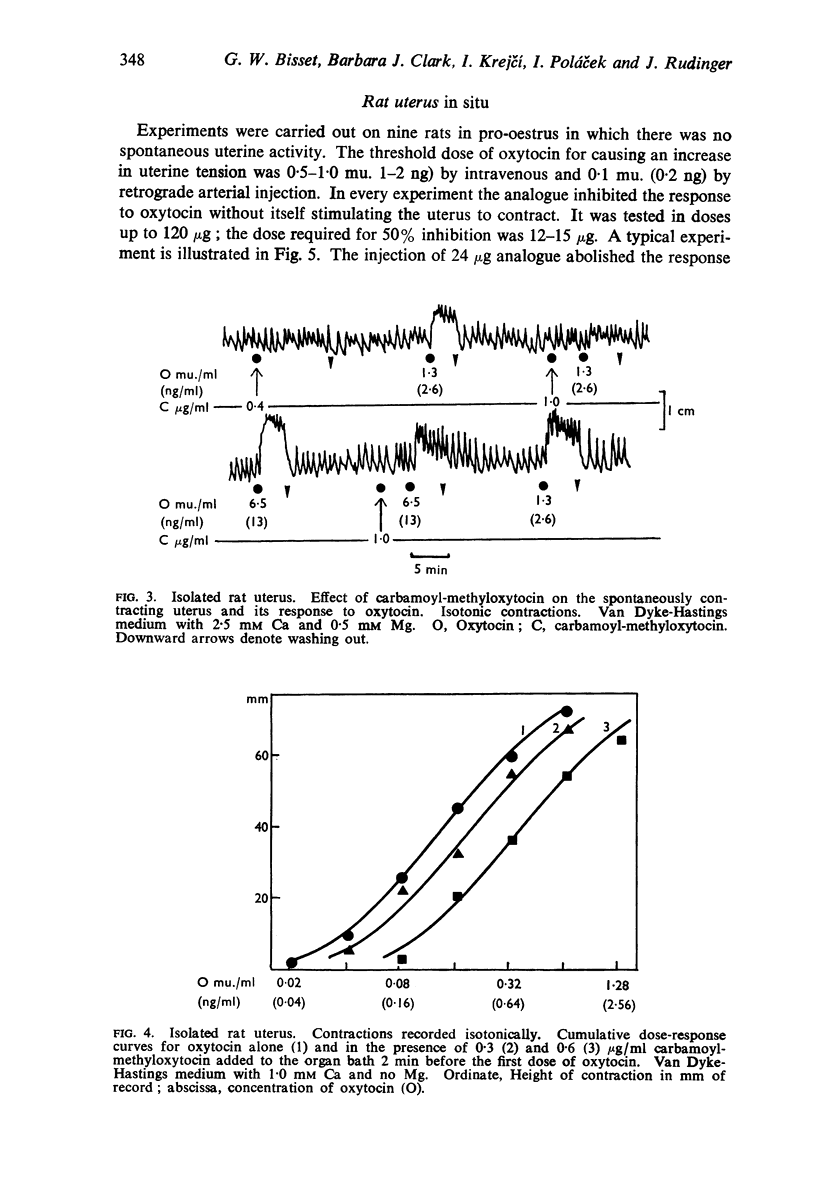
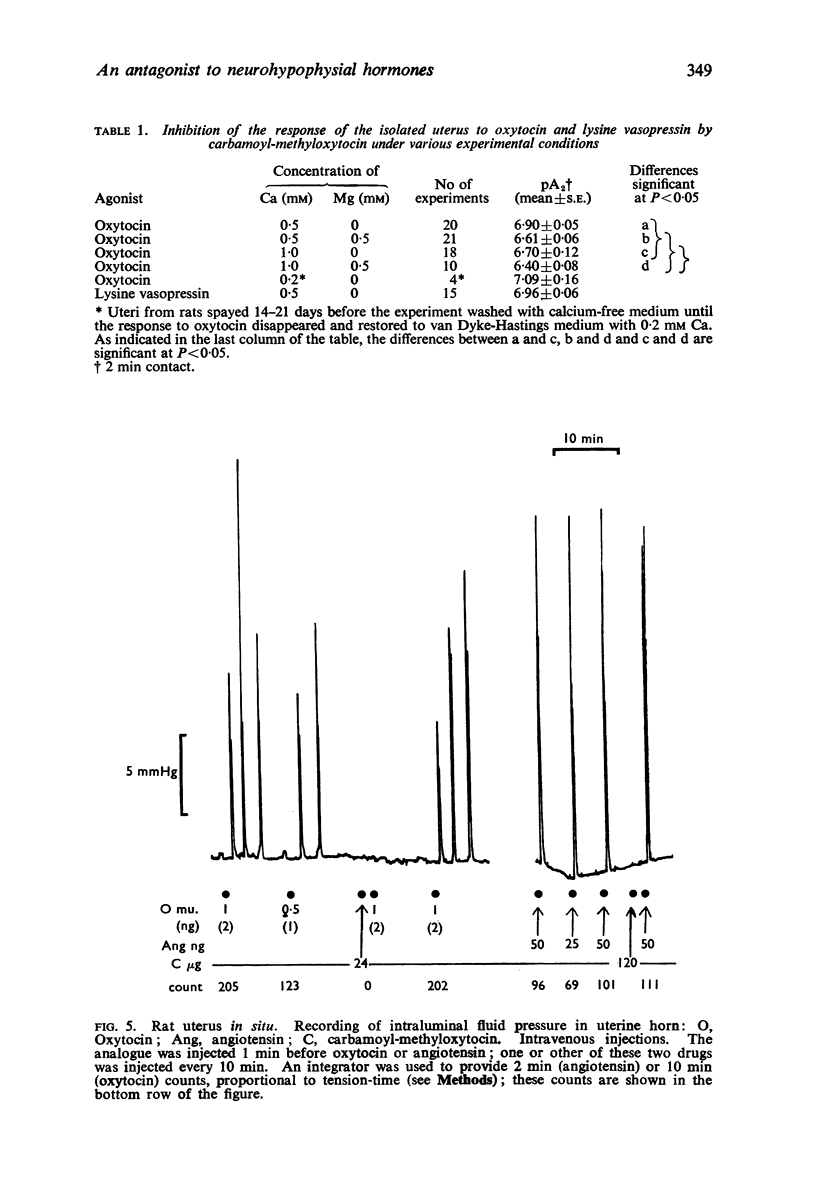
2. The analogue inhibited the response of the isolated rat uterus to both oxytocin and vasopressin without itself stimulating the uterus to contract. The responses to equipotent doses of oxytocin and vasopressin were inhibited equally. There was little or no inhibition of the response to bradykinin. carbachol, angiotensin or 5-hydroxytryptamine with doses of the analogue up to 160 times that required to inhibit the response to oxytocin by 50%. The analogue caused a parallel displacement of the log dose-response curve for oxytocin; the pA2 value (2 min contact) varied from 6·4 to 7·1 according to the ionic composition of the solution in the organ bath.
3. The analogue inhibited the response of the rat uterus in situ to oxytocin but not to angiotensin or 5-hydroxytryptamine. It did not stimulate the uterus.
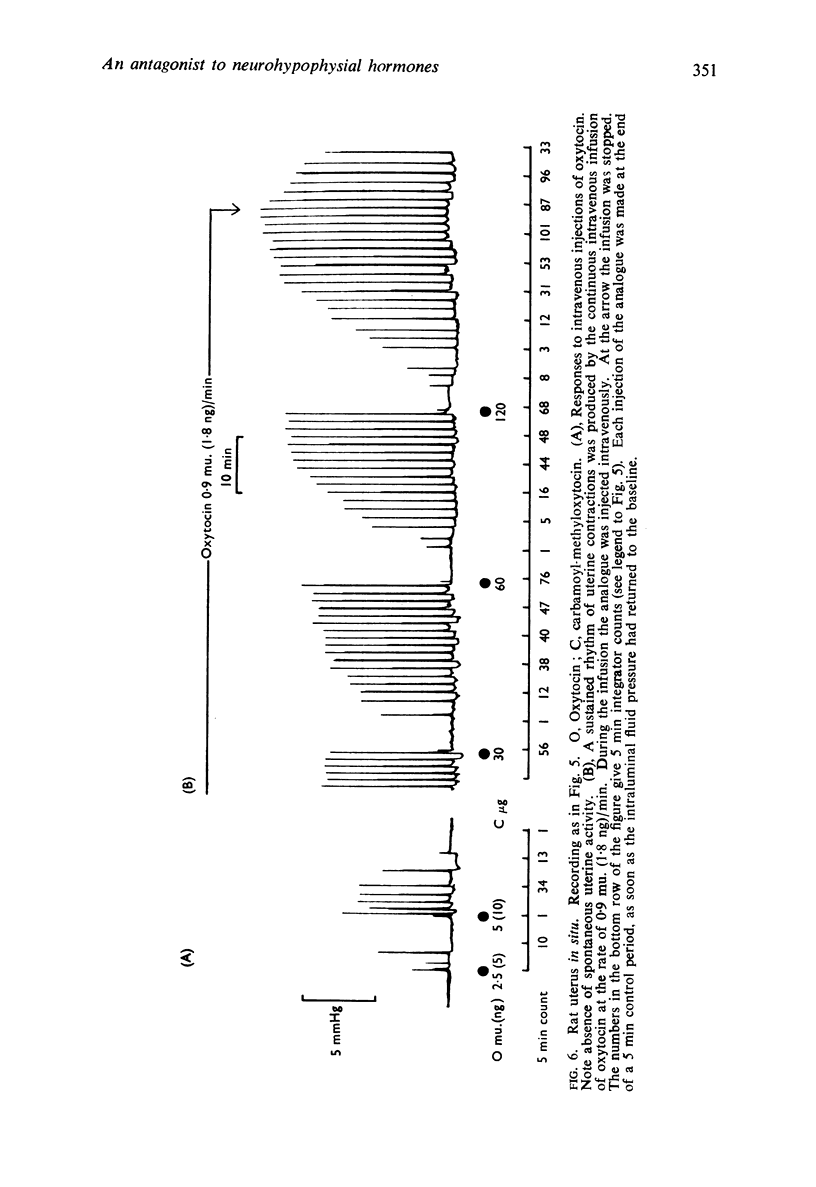
4. When, in certain experimental conditions, spontaneous activity occurred in the isolated uterus or the uterus in situ, this activity was unaffected by the analogue but the increase in amplitude and frequency of contractions caused by oxytocin was inhibited. The regular rhythm of contractions induced in the quiescent uterus by the intravenous infusion of oxytocin was interrupted by intravenous injections of the analogue.
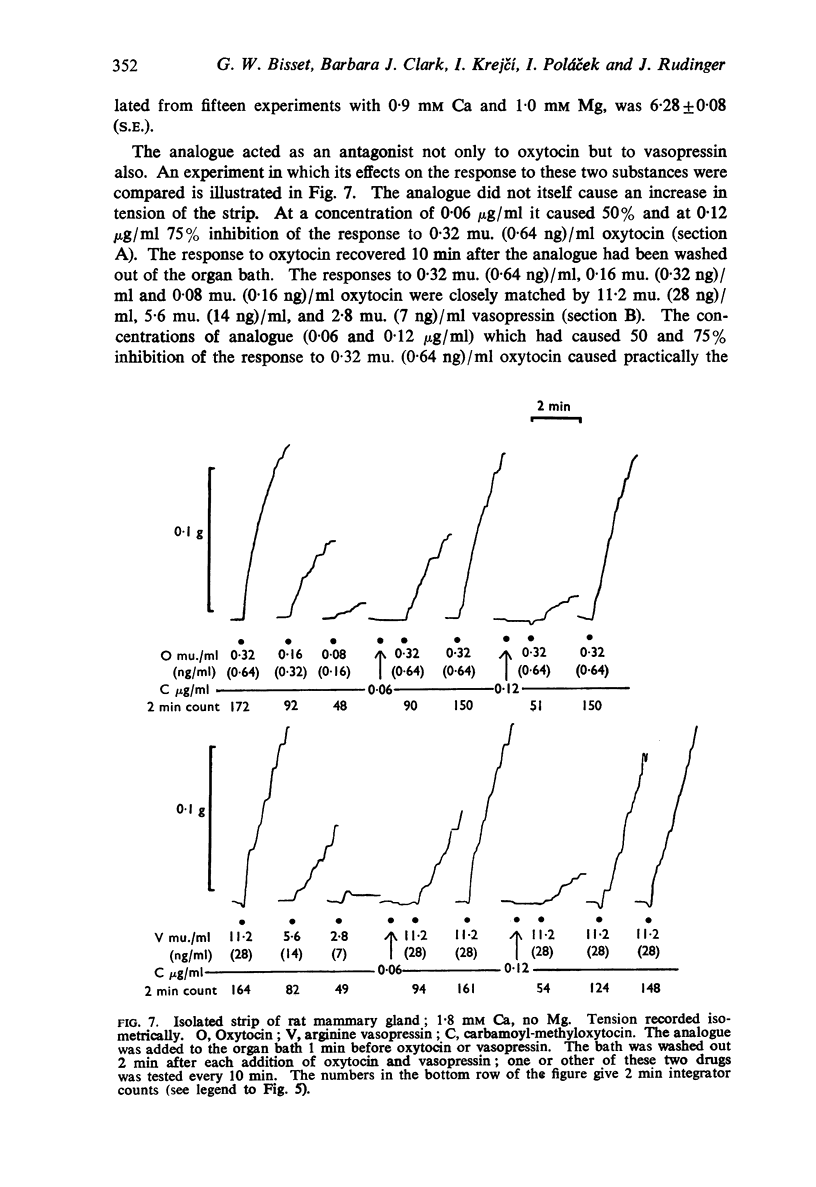
5. The response of the isolated strip of rat mammary gland to the analogue depended on whether or not magnesium was present in the bath solution. In the presence of this ion, the analogue generally caused an increase in tension; in its absence, it acted as a pure antagonist. As on the isolated uterus, oxytocin and vasopressin were equally inhibited, and the analogue caused a parallel displacement of the log dose-response curve for oxytocin. With 0·9 mM Ca and 1·0 mM Mg, the mean pA2 value (2 min contact) was 6·28 ± 0·08 (S.E.)
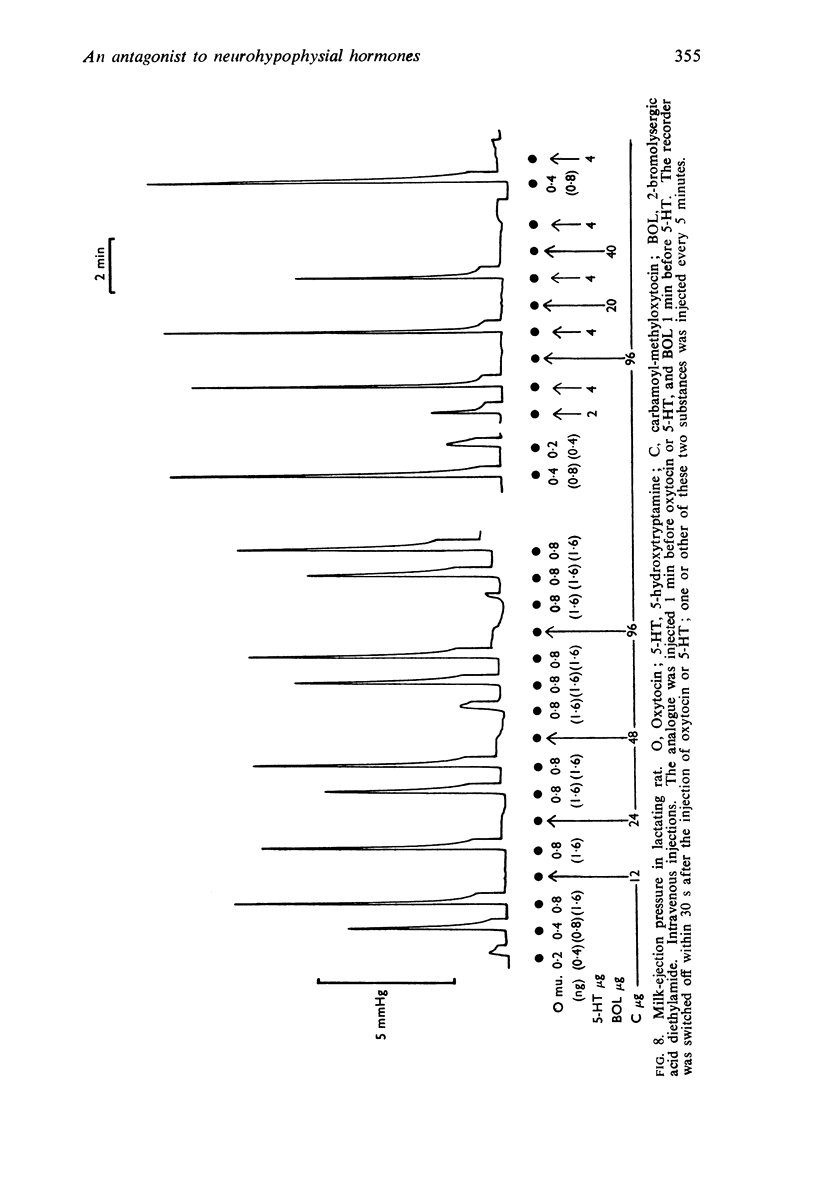
6. In the lactating rat, the analogue inhibited the milk-ejection response to oxytocin and vasopressin but not that to acetylcholine, bradykinin or 5-hydroxytryptamine. A milk-ejection response to the analogue itself was seen occasionally with retrograde arterial but not with intravenous injections.
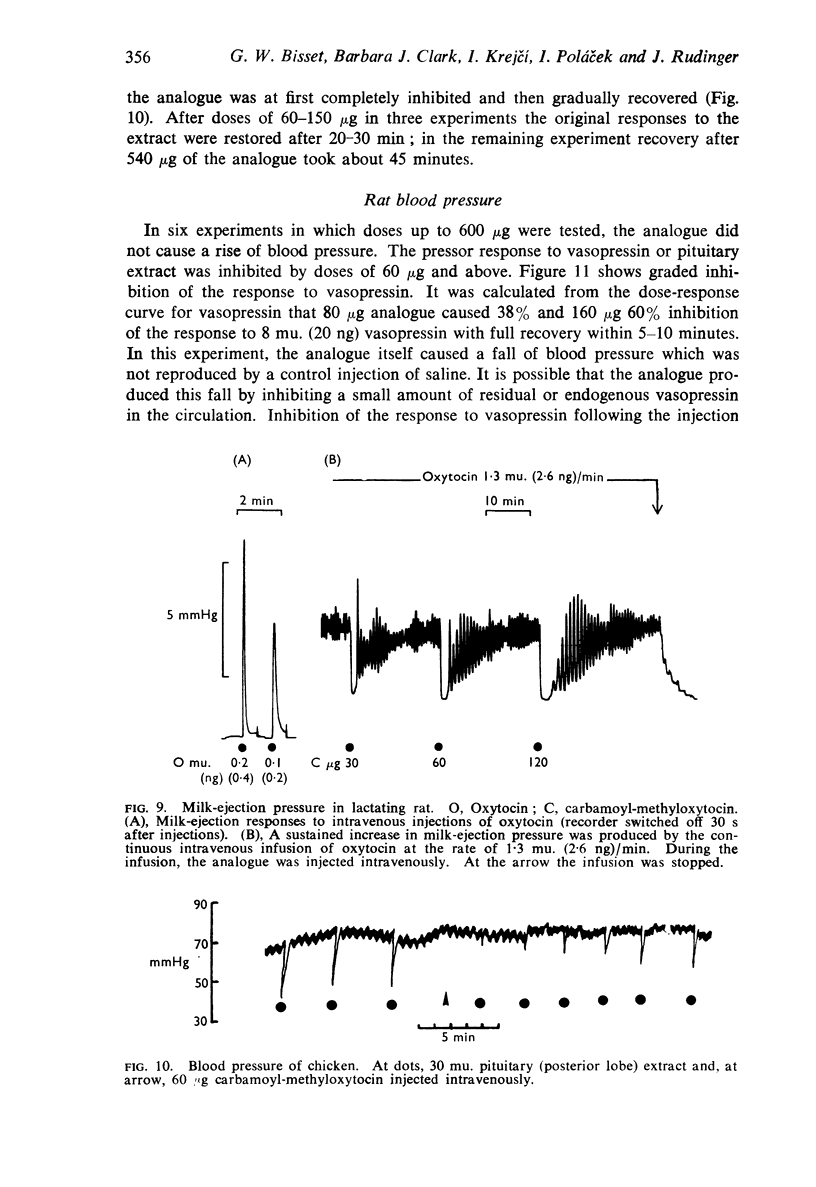
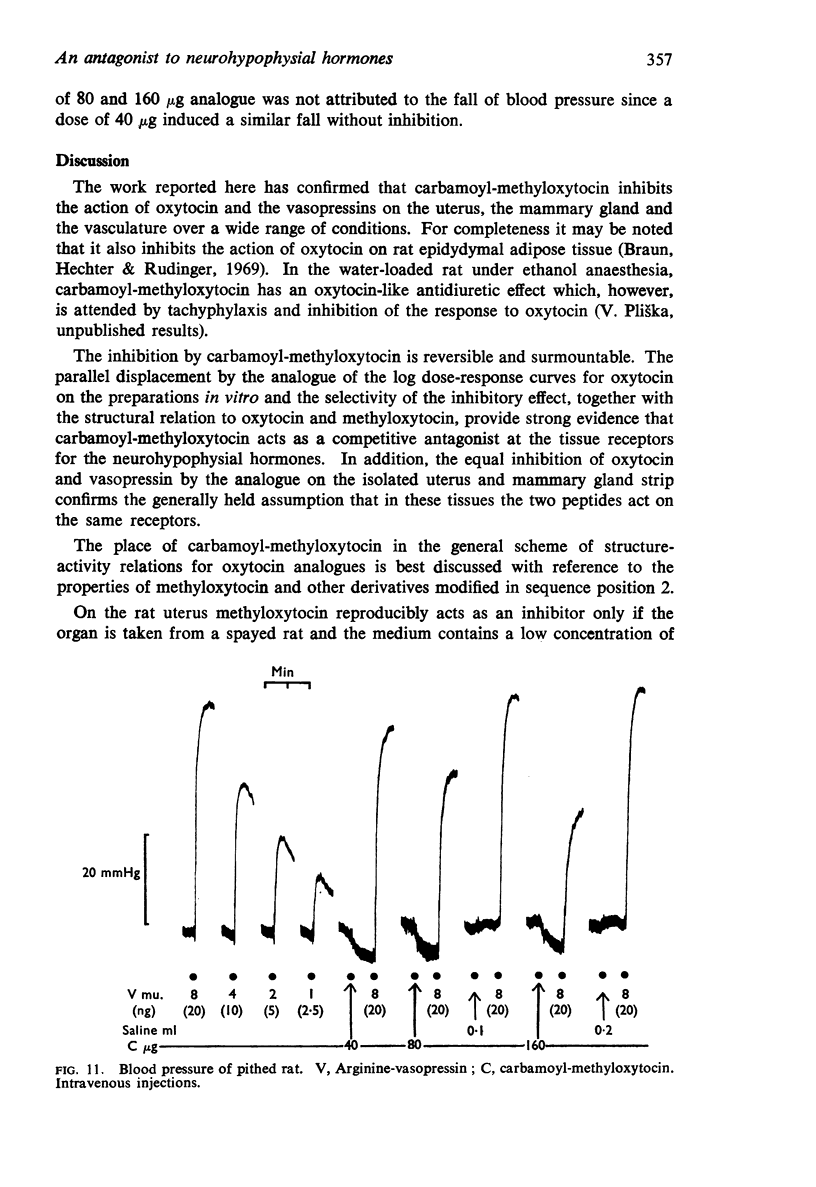
7. The analogue inhibited the avian depressor response to oxytocin and the rat pressor response to vasopressin.
8. On all assay preparations, the degree of inhibition caused by the analogue was dependent on the dose, and the inhibition could be surmounted by increasing the dose of agonist. Recovery usually occurred within 15 min. These features, together with the parallel displacement of the dose-response curve for oxytocin on the isolated uterus and mammary strip, and the equal inhibition of the responses to oxytocin and vasopressin, suggest that carbamoyl-methyl-oxytocin acts as a specific competitive inhibitor of the neurohypophysial hormones.
9. The structure-activity relationships of analogues of oxytocin having substituents in the terminal amino and phenolic hydroxyl groups, and some practical applications of the carbamoyl-methyl analogue, are discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERDE B., CERLETTI A. [On the action of pharmacologic doses of oxytocin on the mammary gland]. Acta Endocrinol (Copenh) 1960 Aug;34:543–557. [PubMed] [Google Scholar]
- Beránková-Ksandrová Z., Bisset G. W., Jost K., Krejci I., Pliska V., Rudinger J., Rychlík I., Sorm F. Synthetic analogues of oxytocin acting as hormonogens. Br J Pharmacol Chemother. 1966 Mar;26(3):615–632. doi: 10.1111/j.1476-5381.1966.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Haldar J. Blood levels of oxytocin and vasopressin during suckling in the rabbit and the problem of their independent release. J Physiol. 1970 Mar;206(3):711–722. doi: 10.1113/jphysiol.1970.sp009039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Haldar J., Harris M. C., Lewis G. P., Rocha e Silva R., Jr The assay of milk-ejecting activity in the lactating rat. Br J Pharmacol Chemother. 1967 Nov;31(3):537–549. doi: 10.1111/j.1476-5381.1967.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J. Synthetic 1-N-carbamylhemicystine-2-O-methyltyrosine-oxytocin (N-carbamyl-O-methyl-oxytocin): a specific antagonist to the actions of oxytocin and vasopressin on the uterus and mammary gland. Nature. 1968 Apr 13;218(5137):197–199. doi: 10.1038/218197b0. [DOI] [PubMed] [Google Scholar]
- Braun T., Hechter O., Rudinger J. "Insulin-like" action of oxytocin: evidence for separate oxytocin-sensitive and insulin-sensitive systems in fat cells. Endocrinology. 1969 Dec;85(6):1092–1096. doi: 10.1210/endo-85-6-1092. [DOI] [PubMed] [Google Scholar]
- CROSS B. A. The motility and reactivity of the oestrogenized rabbit uterus in vivo; with comparative observations on milk ejection. J Endocrinol. 1958 Feb;16(3):237–260. doi: 10.1677/joe.0.0160237. [DOI] [PubMed] [Google Scholar]
- Cort J. H., Rudinger J., Lichardus B., Hagemann I. Effects of oxytocin antagonists on the saluresis accompanying carotid occlusion. Am J Physiol. 1966 Jan;210(1):162–168. doi: 10.1152/ajplegacy.1966.210.1.162. [DOI] [PubMed] [Google Scholar]
- GADDUM J. H., HAMEED K. A., HATHWAY D. E., STEPHENS F. F. Quantitative studies of antagonists for 5-hydroxytryptamine. Q J Exp Physiol Cogn Med Sci. 1955 Jan;40(1):49–74. doi: 10.1113/expphysiol.1955.sp001097. [DOI] [PubMed] [Google Scholar]
- Krejcí I., Kupková B., Vávra I. The effect of some 2-O-alkyltyrosine analogues of oxytocin and lysine vasopressin on the blood pressure of the rat, rabbit, and cat. Br J Pharmacol Chemother. 1967 Aug;30(3):497–505. doi: 10.1111/j.1476-5381.1967.tb02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejcí I., Polácek I., Rudinger J. The action of 2-O-Methyltyrosine-oxytocin on the rat and rabbit uterus: effect of some experimental conditions on change from agonism to antagonism. Br J Pharmacol Chemother. 1967 Aug;30(3):506–517. doi: 10.1111/j.1476-5381.1967.tb02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- Polácek I., Krejcí I., Rudinger J. The action of oxytocin and synthetic analogues on the isolated mammary-gland myoepithelium of the lactating rat; effect of some ions. J Endocrinol. 1967 May;38(1):13–24. doi: 10.1677/joe.0.0380013. [DOI] [PubMed] [Google Scholar]
- RUDINGER J., KREJCI I. Dose-response relations for some synthetic analogues of oxytocin, and the mode of action of oxytocin on the isolated uterus. Experientia. 1962 Dec 15;18:585–588. doi: 10.1007/BF02172197. [DOI] [PubMed] [Google Scholar]
- RYDEN G., SJOHOLM I. Assay of oxytocin by rat mammary gland in vitro. Br J Pharmacol Chemother. 1962 Aug;19:136–141. doi: 10.1111/j.1476-5381.1962.tb01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. G. Acetylation of amino and tyrosine hydroxyl groups. Preparation of inhibitors of oxytocin with no intrinsic activity on the isolated uterus. J Biol Chem. 1967 Apr 10;242(7):1592–1598. [PubMed] [Google Scholar]
- Smyth D. G. Carbamylation of amino and tyrosine hydroxyl groups. Preparation of an inhibitor of oxytocin with no intrinsic activity on the isolated uterus. J Biol Chem. 1967 Apr 10;242(7):1579–1591. [PubMed] [Google Scholar]
- Smyth D. G. On the molecular mechanism of oxytocin action. Biochim Biophys Acta. 1970 Feb 17;200(2):395–403. doi: 10.1016/0005-2795(70)90182-0. [DOI] [PubMed] [Google Scholar]
- Yamashiro D., Aanning H. L., Branda L. A., Cash W. D., Murti V. V., du Vigneaud V. A synthesis of [1-(N-methyl-hemi-L-cystine)]-oxytocin and a study of its reaction with acetone. J Am Chem Soc. 1968 Jul 17;90(15):4141–4144. doi: 10.1021/ja01017a040. [DOI] [PubMed] [Google Scholar]


