Abstract
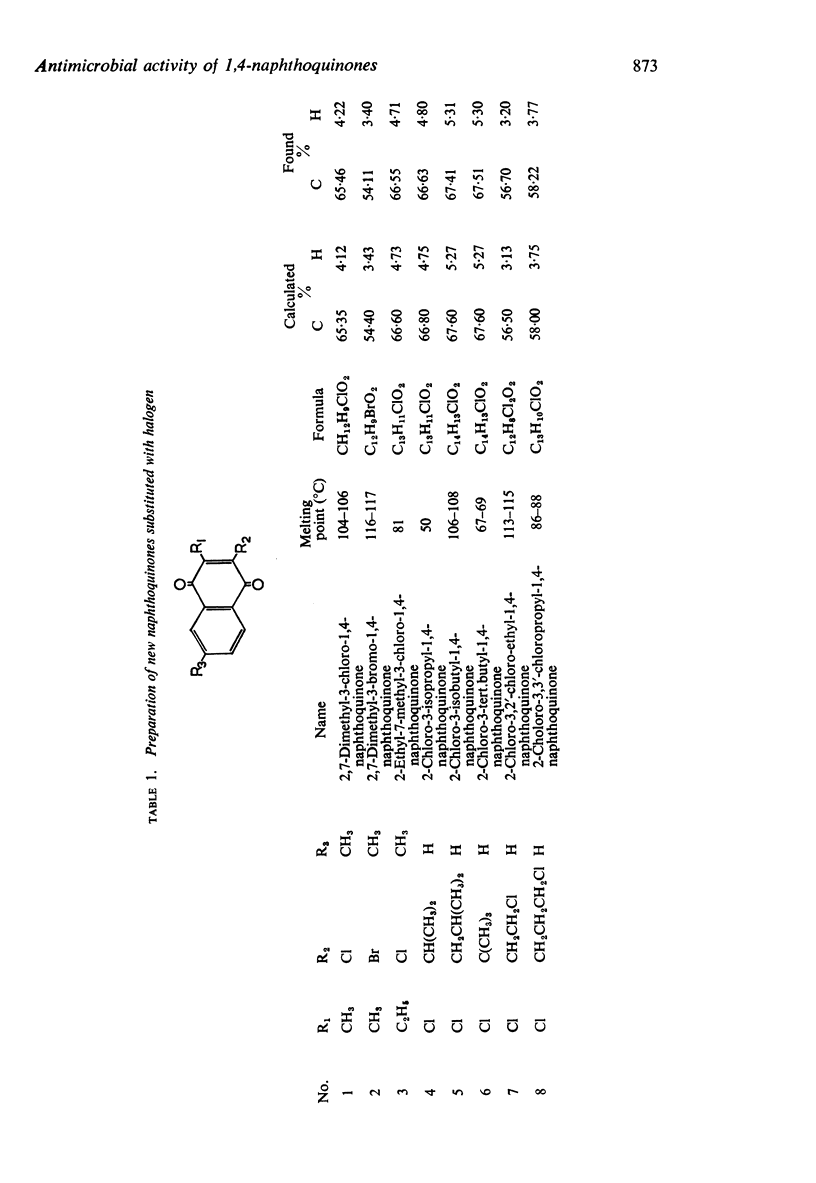
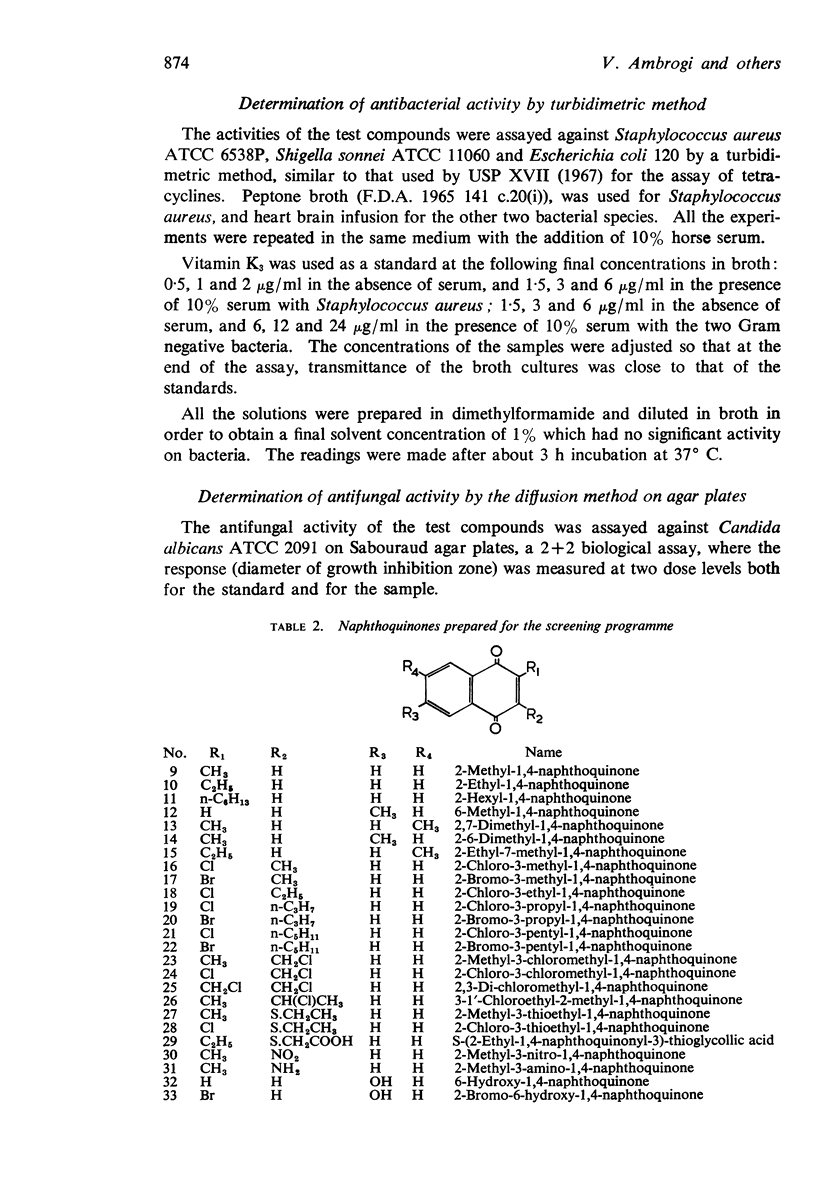
1. New halogenated 1,4-naphthoquinones were synthesized and together with other known 1,4-naphthoquinones, were screened for antibacterial activity by a turbidimetric method, and for antifungal activity by the diffusion method on agar plates.
2. The half-wave potentials and the influence on the oxidative phosphorylation of some of these compounds were determined.
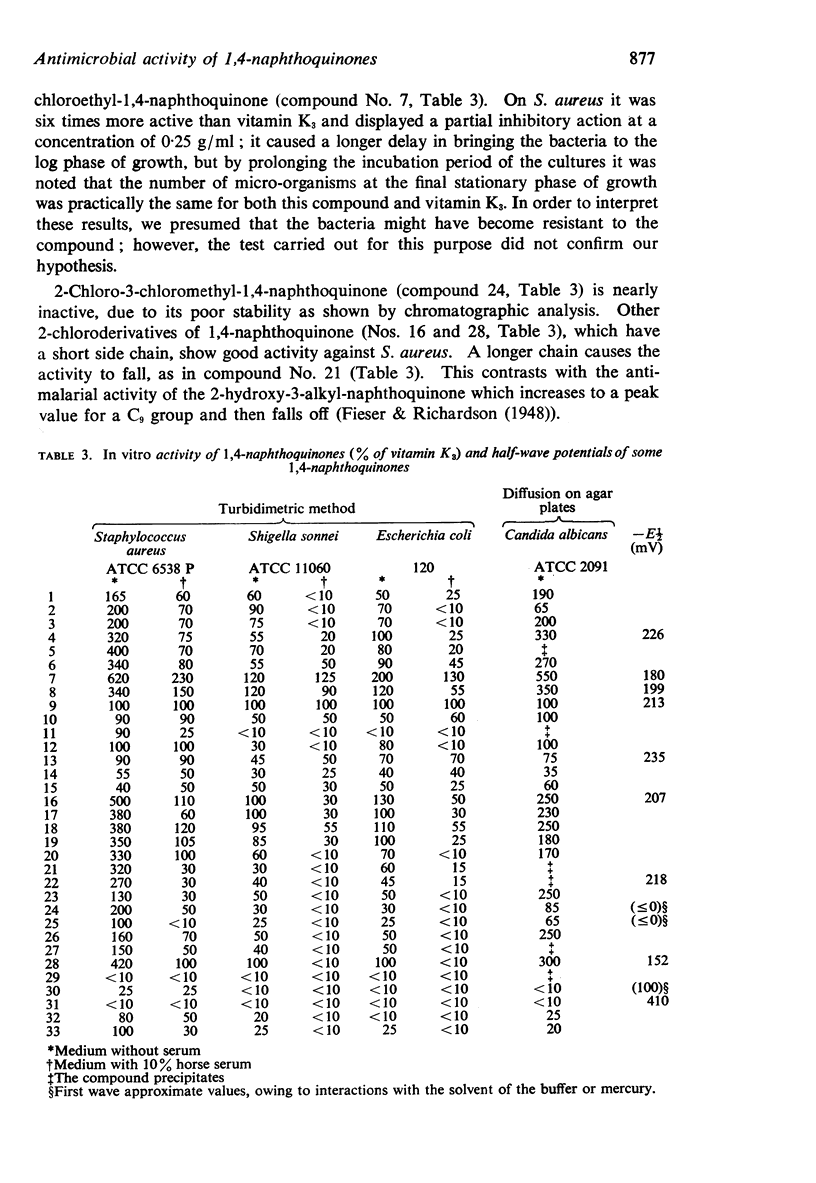
3. 2-chloro-3,2′-chloro-ethyl-1,4-naphthoquinone (half-wave potential=-187 mV) was the most active compound, completely inhibiting cell respiration.
4. While the natural active naphthoquinones, vitamin K and ubiquinones, possess, as substituent, the electron repelling methyl group, the microbiologically active 1,4-naphthoquinones are substituted, in the quinone moiety, with electron attracting groups such as OH or Cl.
5. The half-wave potentials can give only an initial indication of the activity of the compounds studied; a good correlation, on the contrary, can be found between the ultraviolet spectra of such compounds and their activity which seems to depend on the ability of active compounds to exist in an extensively conjugated structure and to form hydrogen bonds.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKIYA S. Studies on synthetic compounds active against Salmonella-dysentery group bacilli. Jpn J Exp Med. 1956 Aug;26(3-4):91–112. [PubMed] [Google Scholar]
- Bullock F. J. Antiprotozoal quinones. I. Synthesis of 2-hydroxy-3-alkyl-1,4-naphthoquinones as potential coccidiostats. J Med Chem. 1968 May;11(3):419–424. doi: 10.1021/jm00309a002. [DOI] [PubMed] [Google Scholar]
- Colwell C. A., McCall M. The Mechanism of Bacterial and Fungus Growth Inhibition by 2-Methyl-1,4-Naphthoquinone. J Bacteriol. 1946 Jun;51(6):659–670. doi: 10.1128/jb.51.6.659-670.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes H. L., Currie D. J., Maltman J. R., Silver R. F., Lough C. E., Leahy G. J. Evidence for the mode of chemical action of 1,4-naphthoquinones in bacteriostasis. Chemotherapy. 1964;9(4):241–247. doi: 10.1159/000220373. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. Oxidative phosphorylations; rôle of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952 Mar;195(1):215–224. [PubMed] [Google Scholar]
- MARTIUS C., NITZ-LITZOW D. Uber den Wirkungsmechanismus des Dicumarols und verwandter Verbindungen. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):134–140. doi: 10.1016/0006-3002(53)90132-2. [DOI] [PubMed] [Google Scholar]
- Redfearn E. R., Burgos J. Ubiquinone (coenzyme Q) and the respiratory chain. Nature. 1966 Feb 12;209(5024):711–713. doi: 10.1038/209711a0. [DOI] [PubMed] [Google Scholar]


