Abstract
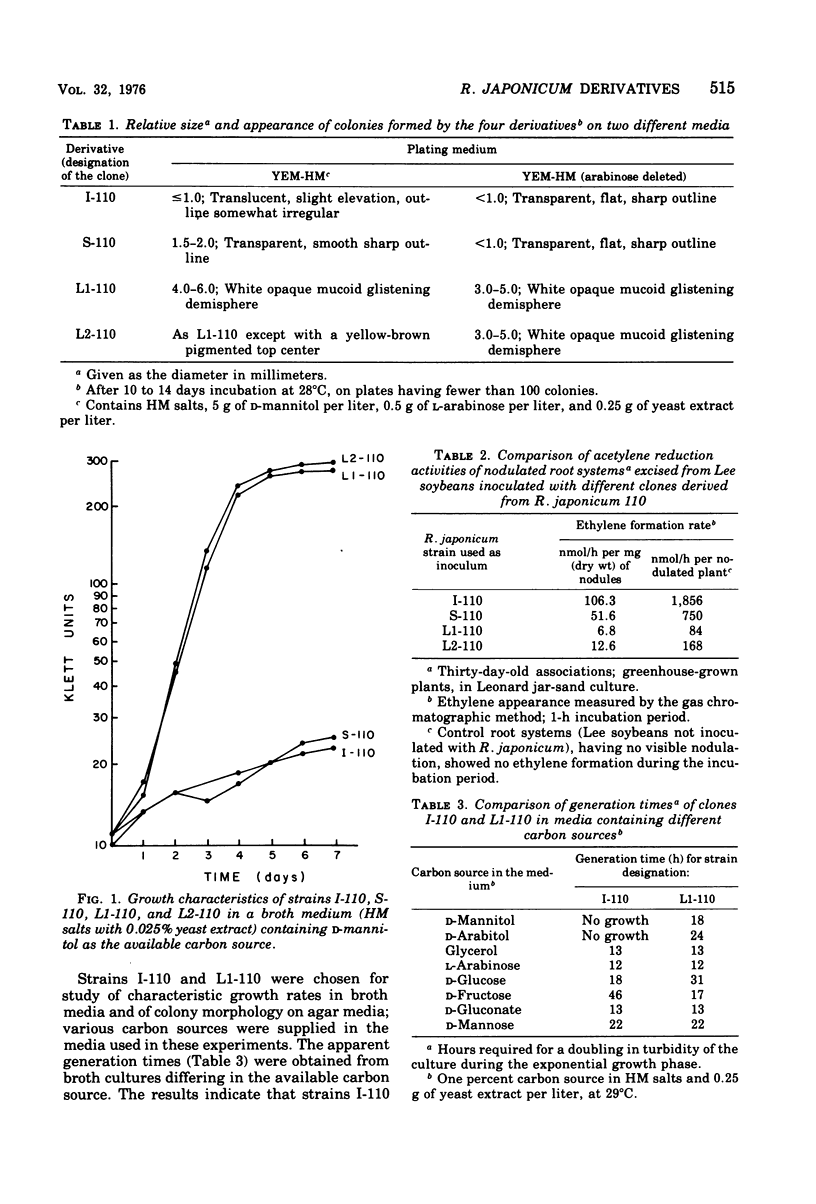
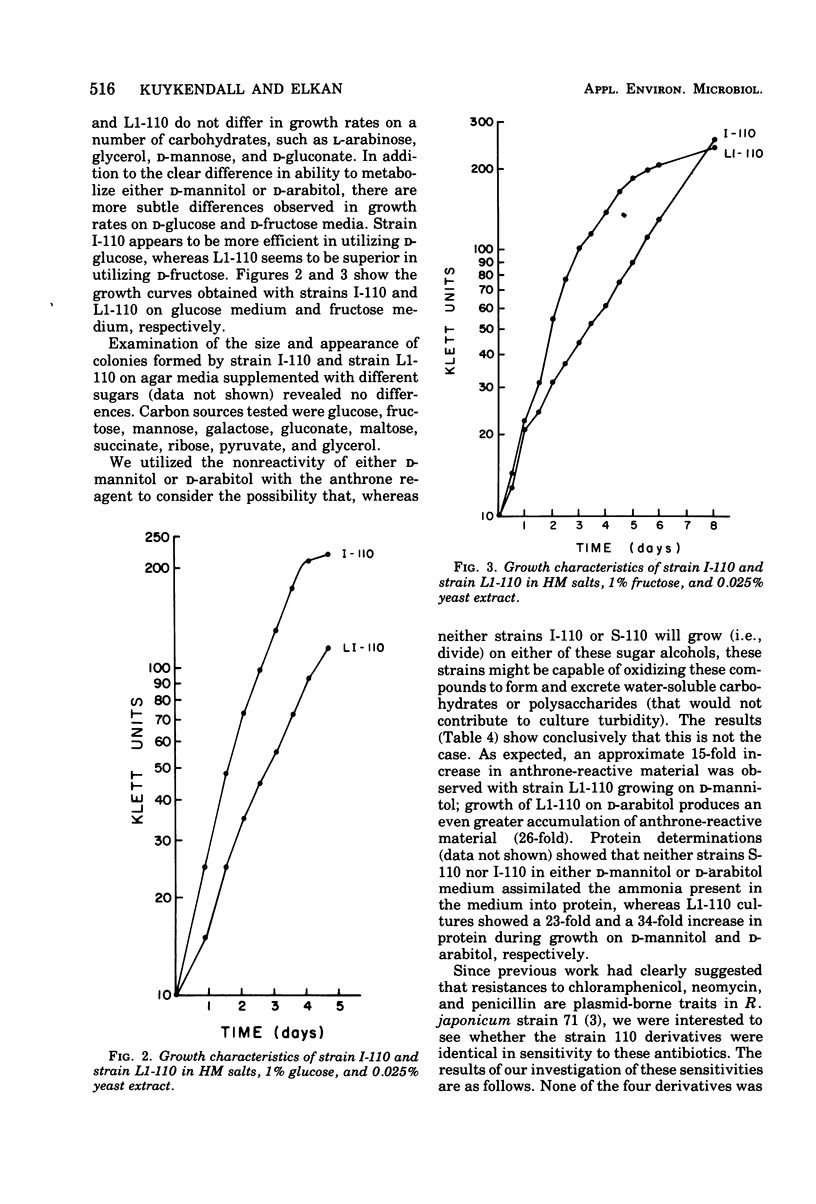
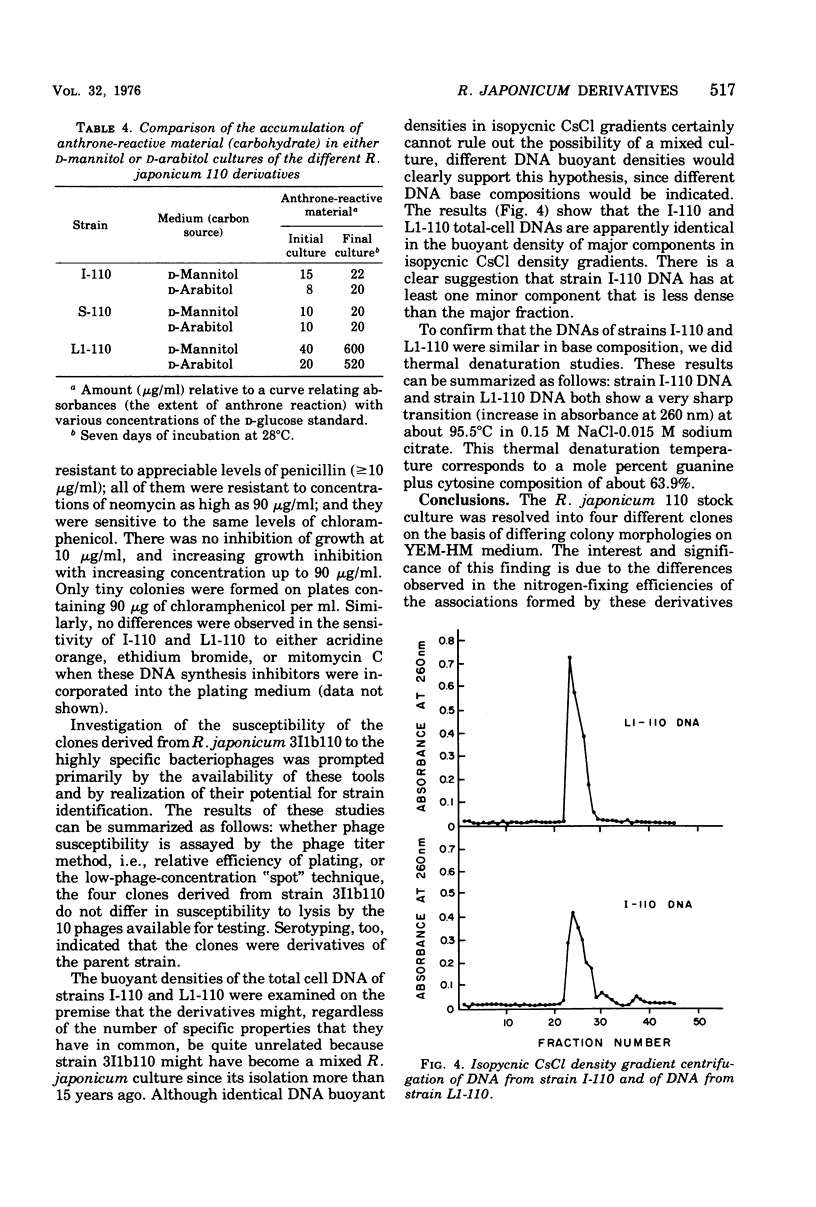
Four derivatives of Rhizobium japonicum 110 were isolated on the basis of morphologically different colonies formed on yeast extract-mannitol-HM salts medium. All are able to nodulate Lee soybeans. The bacteria-plant associations formed by each clone have measurable acetylene-reducing activity, but those formed by two of these clones (designated L1-110 and L2-110) are 5- to 10-fold less efficient than those formed by the others (designated I-110 and S-110). These derivatives were not detectable with ordinary culture techniques since, because of cell adherence, genetically mixed colonies result. When a detergent (Tween 40 at 0.01%, vol/vol) was added to the dilution medium, separate clones resulted. The metabolic basis for the gross differences in colony morphology on yeast extract-mannitol-HM salts medium was found to be that L1-110 and L2-110 utilized p-mannitol for growth, whereas I-110 and S-110 did not. These clones differ analogously in ability to utilize D-arabitol. Clones I-110 and L1-110 were chosen for studies of growth rates on various carbohydrates. Although clone I-110 and clone L1-110 did not differ in growth rates on a number of sugars, such as gluconate, arabinose, glycerol, and mannose, they differed in growth rates on glucose and fructose. Although clone I-110 grew faster on glucose than did clone L1-110, clone L1-110 grew faster on fructose than did clone I-110. Clones I-110 and L1-110 showed identical responses to several antibiotics and deoxyribonucleic acid (DNA) synthesis inhibitors and identical susceptibility to some highly specific bacteriophages. Identical buoyant densities of their DNAs in isopycnic CsCl density gradients and identical thermal denaturation temperatures of their DNAs suggest that clones I-110 and L1-110 have the same DNA base composition. Preliminary DNA/DNA hybridization experiments show that strain I-110 DNA and strain L1-110 DNA have a high degree of common polynucleotide sequences.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. K., ALLEN O. N. Biochemical and symbiotic properties of the rhizobia. Bacteriol Rev. 1950 Dec;14(4):273–330. doi: 10.1128/br.14.4.273-330.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. A., Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973 Sep;4(3):248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L. Acetylene reduction by pure cultures of Rhizobia. J Bacteriol. 1975 Sep;123(3):1265–1268. doi: 10.1128/jb.123.3.1265-1268.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larue T. A., Kurz W. G. Estimation of nitrogenase using a colorimetric determination for ethylene. Plant Physiol. 1973 Jun;51(6):1074–1075. doi: 10.1104/pp.51.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillich T. T., Elkan G. H. Evidence countering the role of polygalacturonase in invasion of root hairs of leguminous plants by Rhizobium spp. Can J Microbiol. 1968 Jun;14(6):617–625. doi: 10.1139/m68-104. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Martínez-De Drets G., Arias A. Enzymatic basis for differentiation of Rhizobium into fast- and slow-growing groups. J Bacteriol. 1972 Jan;109(1):467–470. doi: 10.1128/jb.109.1.467-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Sloger C. Symbiotic effectiveness and n(2) fixation in nodulated soybean. Plant Physiol. 1969 Dec;44(12):1666–1668. doi: 10.1104/pp.44.12.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. Some features of the DNA of Rhizobium bacteroids and bacteria. Biochim Biophys Acta. 1974 Sep 27;366(1):1–10. doi: 10.1016/0005-2787(74)90312-8. [DOI] [PubMed] [Google Scholar]
- Tjepkema J., Evans H. J. Nitrogen fixation by free-living Rhizobium in a defined liquid medium. Biochem Biophys Res Commun. 1975 Jul 22;65(2):625–628. doi: 10.1016/s0006-291x(75)80192-6. [DOI] [PubMed] [Google Scholar]



