Abstract
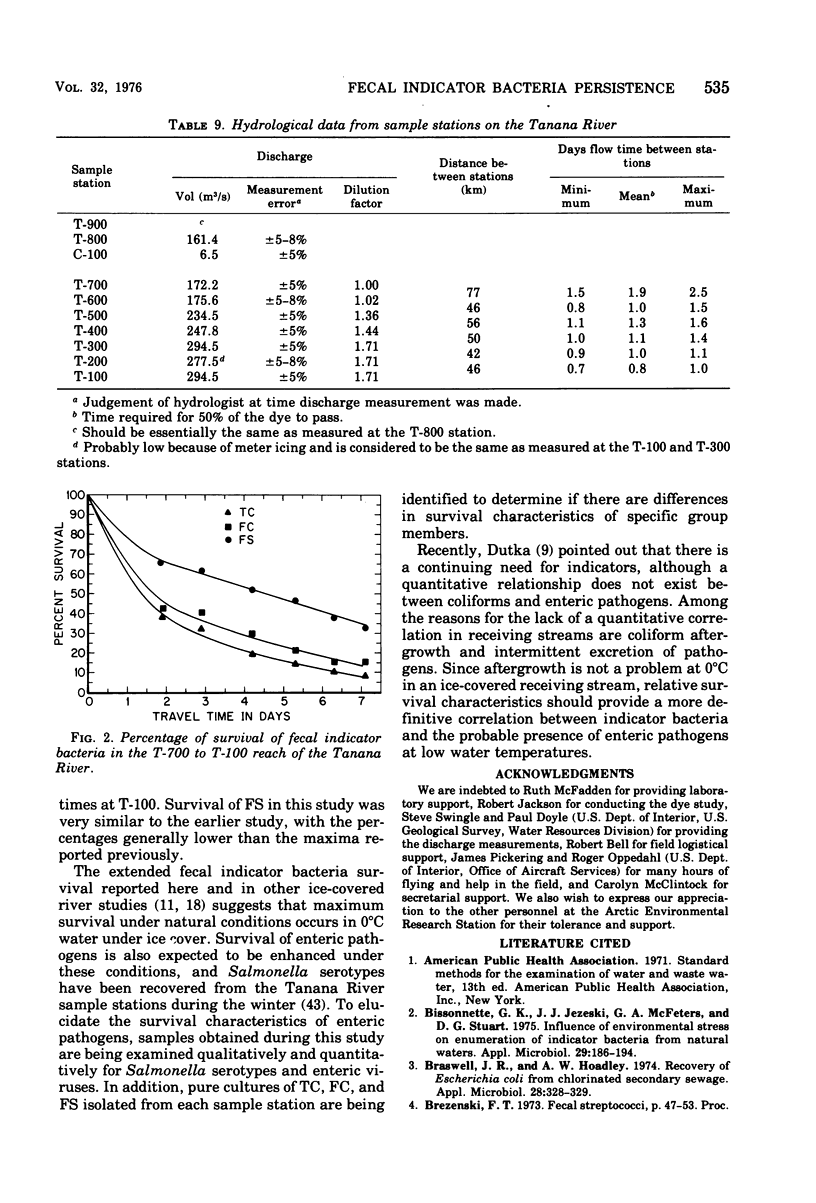
Total coliform (TC), fecal coliform (FC), and fecal streptococcus (FS) survival characteristics, under natural conditions at 0 degrees C in an ice-covered river, were examined during February and March 1975. The membrane filter (MF) technique was used throughout the study, and the multiple-tube (MPN) method was used in parallel on three preselected days for comparative recovery of these bacteria. Survival was studied at seven sample stations downstream from all domestic pollution sources in a 317-km reach of the river having 7.1 days mean flow time (range of 6.0 to 9.1 days). The mean indicator bacteria densities decreased continuously at successive stations in this reach and, after adjustment for dilution, the most rapid die-off was found to occur during the first 1.9 days, followed by a slower decrease. After 7.1 days, the relative survival was TC less than FC less than FS, with 8.4%, 15.7%, and 32.8% of the initial populations remaining viable, respectively. These rates are higher than previously reported and suggest that the highest survival rates for these bacteria in receiving streams can be expected at 0 degree C under ice cover. Additionally, the FC-FS ratio was greater than 5 at all stations, indicating that this ratio may be useable for determining the source of fecal pollution in receiving streams for greater than 7 days flow time at low water temperatures. The MPN and MF methods gave comparable results for the TC and FS at all seven sample stations, with both the direct and verified MF counts within the 95% confidence limits of the respective MPNs in most samples, but generally lower than the MPN index. Although FC recovery on membrane filters was comparable results at stations near the pollution source. However, the results became more comparable with increasing flow time. The results of this study indicate that heat shock is a major factor in suppression of the FC counts on the membrane filters at 44.5 degree C. Heat shock may be minimized by extended incubation at 35 degrees C before exposure to the higher temperature.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braswell J. R., Hoadley A. W. Recovery of Escherichia coli from chlorinated secondary sewage. Appl Microbiol. 1974 Aug;28(2):328–329. doi: 10.1128/am.28.2.328-329.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoust R. A., Litsky W. Pfizer selective enterococcus agar overlay method for the enumeration of fecal streptococci by membrane filtration. Appl Microbiol. 1975 May;29(5):584–589. doi: 10.1128/am.29.5.584-589.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka B. J., Jackson M. J., Bell J. B. Comparison of autoclave and ethylene oxide-sterilized membrane filters used in water quality studies. Appl Microbiol. 1974 Sep;28(3):474–480. doi: 10.1128/am.28.3.474-480.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldreich E. E., Best L. C., Kenner B. A., Van Donsel D. J. The bacteriological aspects of stormwater pollution. J Water Pollut Control Fed. 1968 Nov;40(11):1861–1872. [PubMed] [Google Scholar]
- Hoadley A. W., Cheng C. M. The recovery of indicator bacteria on selective media. J Appl Bacteriol. 1974 Mar;37(1):45–57. doi: 10.1111/j.1365-2672.1974.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Hufham J. B. Evaluating the membrane fecal coliform test by using Escherichia coli as the indicator organism. Appl Microbiol. 1974 Apr;27(4):771–776. doi: 10.1128/am.27.4.771-776.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNER B. A., CLARK H. F., KABLER P. W. Fecal Streptococci. I. Cultivation and enumeration of Streptococci in surface waters. Appl Microbiol. 1961 Jan;9:15–20. doi: 10.1128/am.9.1.15-20.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNER B. A., CLARK H. F., KABLER P. W. Fecal Streptococci. II. Quantification of Streptococci in feces. Am J Public Health Nations Health. 1960 Oct;50:1553–1559. doi: 10.2105/ajph.50.10.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Evaluation of coliform tests for chlorinated secondary effluents. J Water Pollut Control Fed. 1973 Mar;45(1):498–506. [PubMed] [Google Scholar]
- Lin S., Evans R. L., Beuscher D. B. Bacteriological assessment of Spoon River water quality. Appl Microbiol. 1974 Aug;28(2):288–297. doi: 10.1128/am.28.2.288-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Bissonnette G. K., Jezeski J. J., Thomson C. A., Stuart D. G. Comparative survival of indicator bacteria and enteric pathogens in well water. Appl Microbiol. 1974 May;27(5):823–829. doi: 10.1128/am.27.5.823-829.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presswood W. G., Brown L. R. Comparison of Gelman and Millipore membrane filters for enumerating fecal coliform bacteria. Appl Microbiol. 1973 Sep;26(3):332–336. doi: 10.1128/am.26.3.332-336.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E., Geldreich E. E., Litsky W. Improved membrane filter method for fecal coliform analysis. Appl Microbiol. 1975 Apr;29(4):532–536. doi: 10.1128/am.29.4.532-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer D. J., Long M. C., Janardan K. G. Statistical analysis of the recovery of coliform organisms on Gelman and Millipore membrane filters. Appl Microbiol. 1974 Oct;28(4):605–607. doi: 10.1128/am.28.4.605-607.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L. J., Morrison S. M., Mayeux J. V. Synergistic false-positive coliform reaction on M-Endo MF medium. Appl Microbiol. 1970 Nov;20(5):778–781. doi: 10.1128/am.20.5.778-781.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. K., Marr A. G., Ingraham J. L. Determination of the minimal temperature for growth of Escherichia coli. J Bacteriol. 1971 Feb;105(2):683–684. doi: 10.1128/jb.105.2.683-684.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Donsel D. J., Geldreich E. E., Clarke N. A. Seasonal Variations in Survival of Indicator Bacteria in Soil and Their Contribution to Storm-water Pollution. Appl Microbiol. 1967 Nov;15(6):1362–1370. doi: 10.1128/am.15.6.1362-1370.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



