Abstract
1. Preparations bathed in a well stirred solution have been considered as heterogeneous systems in which the solid phase is enveloped by a thin layer of stationary liquid. Any substance applied into the bulk solution must pass through this layer by diffusion before reaching the receptors.
2. The rate of diffusion through the stationary layer can govern the time course of the cellular responses to applied drugs provided that (i) all receptors involved in the response are situated at an equal distance from the solution and (ii) interaction with the receptor and consequent cellular events are very rapid.
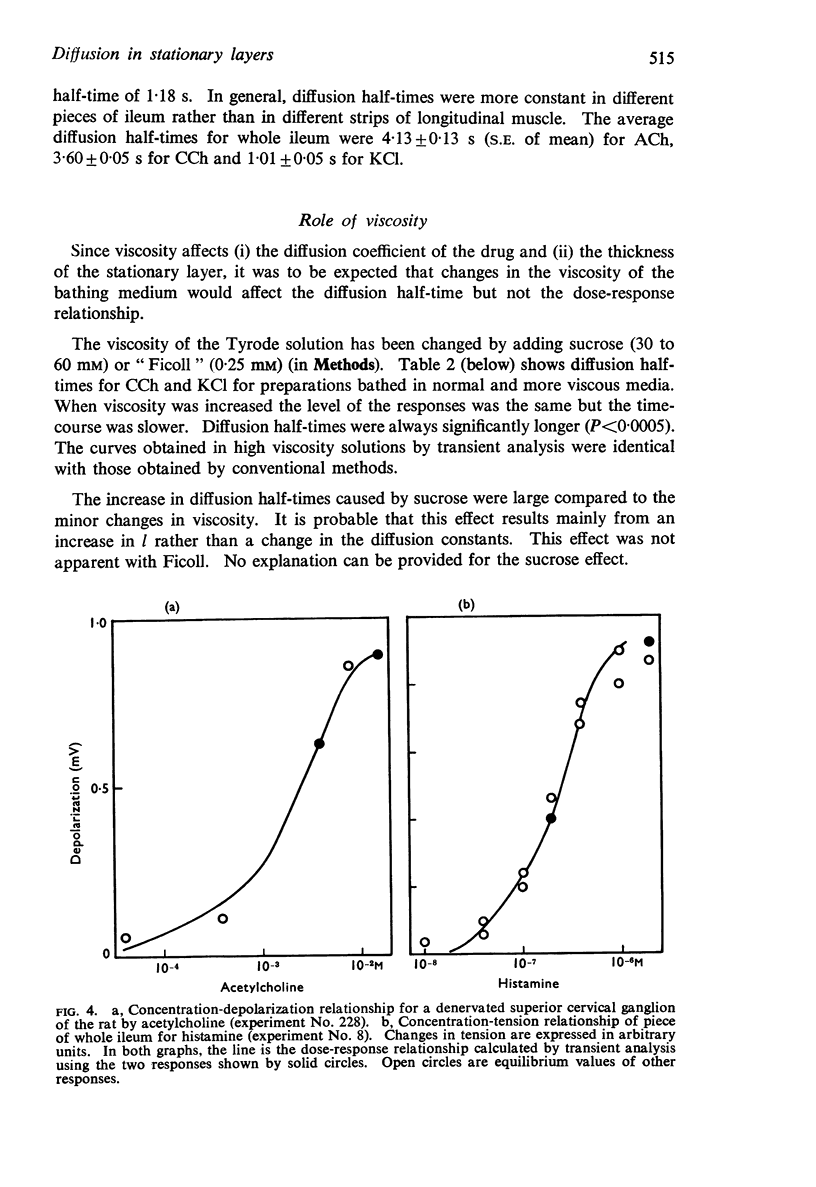
3. These conditions have been verified for two responses: the contraction of guinea-pig ileum by acetylcholine (ACh), carbamylcholine (CCh), histamine and KCl, and the depolarization of the rat isolated sympathetic ganglion by ACh in the presence of eserine. A method of analysis has been applied which allows a complete dose-response curve to be obtained from only two responses.
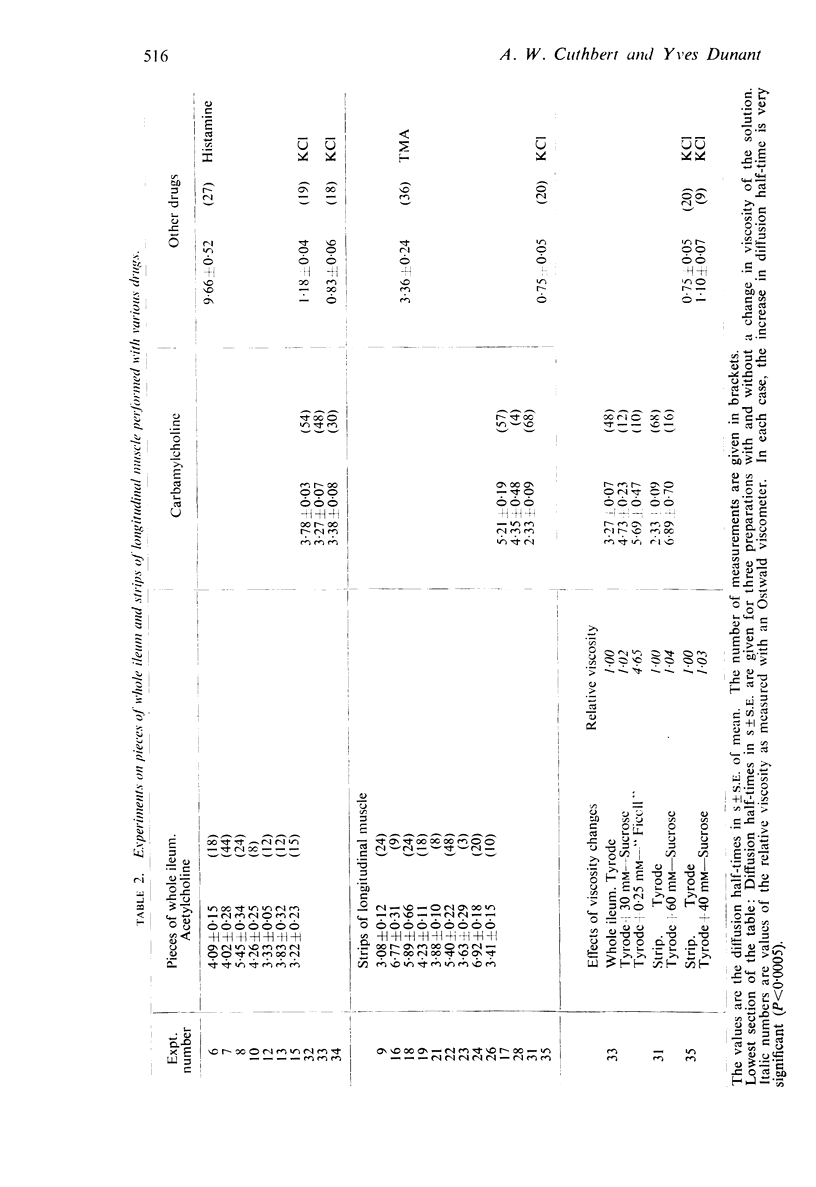
4. Diffusion half-times measured for pieces of ileum were 4·13 ± 0·13 s (S.E. of mean) for Ach, 3·60 ± 0·05 s for CCh and 1·01 + 0·05 s for KCl. The equivalent thickness of the stationary layer calculated from these values was respectively 93 μm, 87 μm and 70 μm. The average diffusion half-time for ACh in sympathetic ganglia was 14·19 ± 1·05 s. This gives an equivalent thickness of 173 μm.
5. Diffusion half-times were increased by increasing the viscosity of the bathing solution without changing the concentration response relationship.
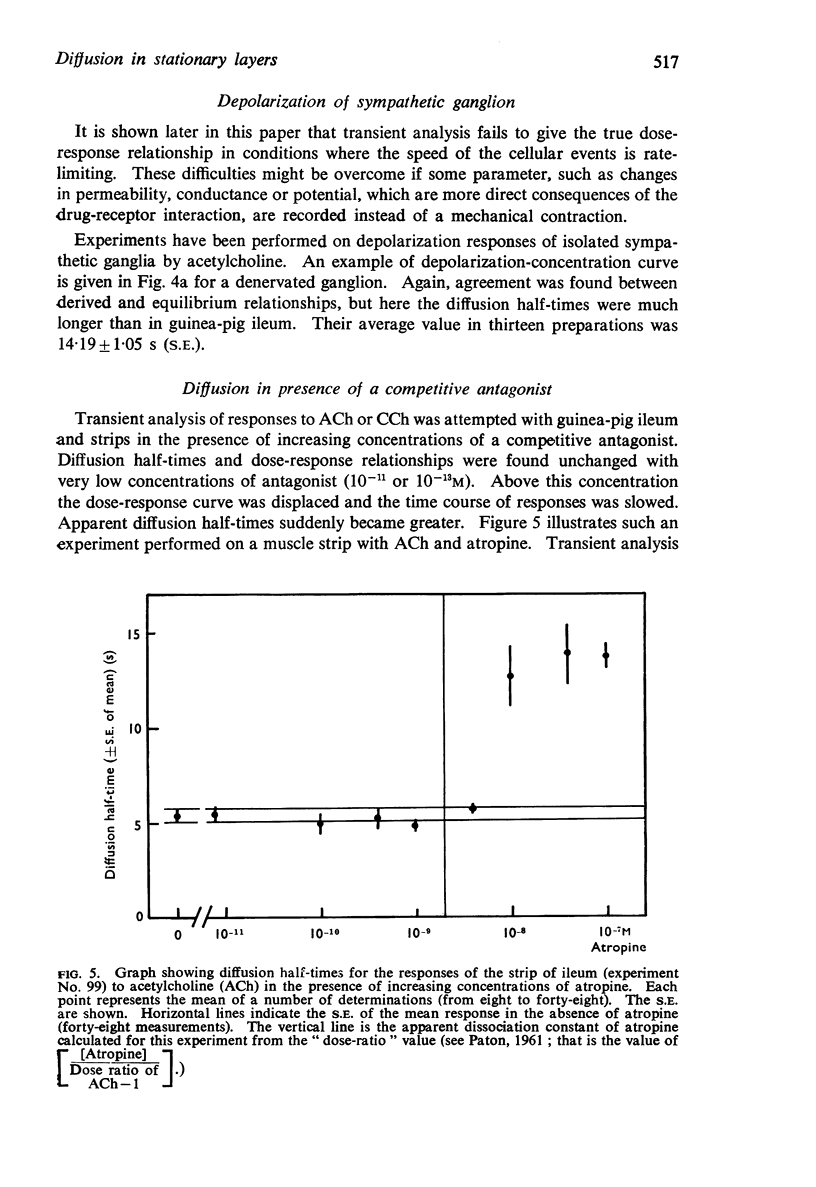
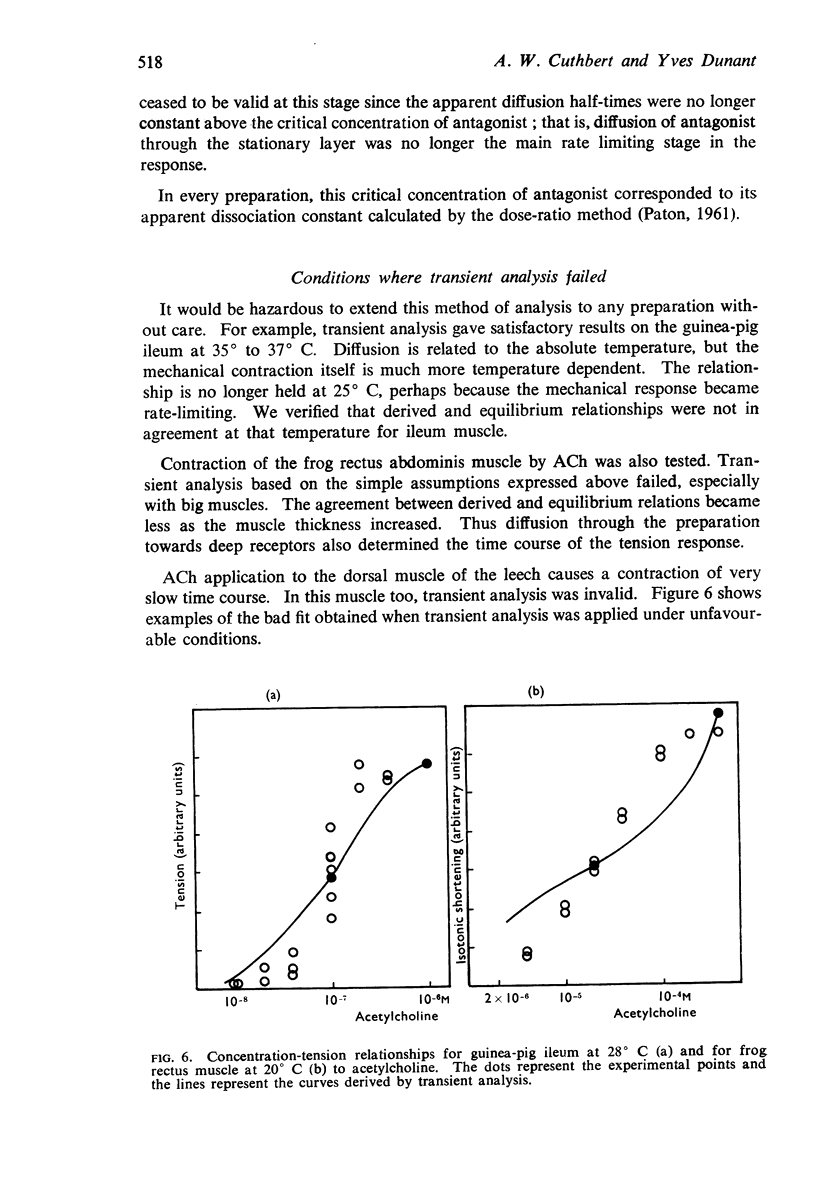
6. The time course of contractions of guinea-pig ileum are no longer diffusion limited in the presence of a competitive antagonist or when the temperature is lowered from 35° to 25° C.
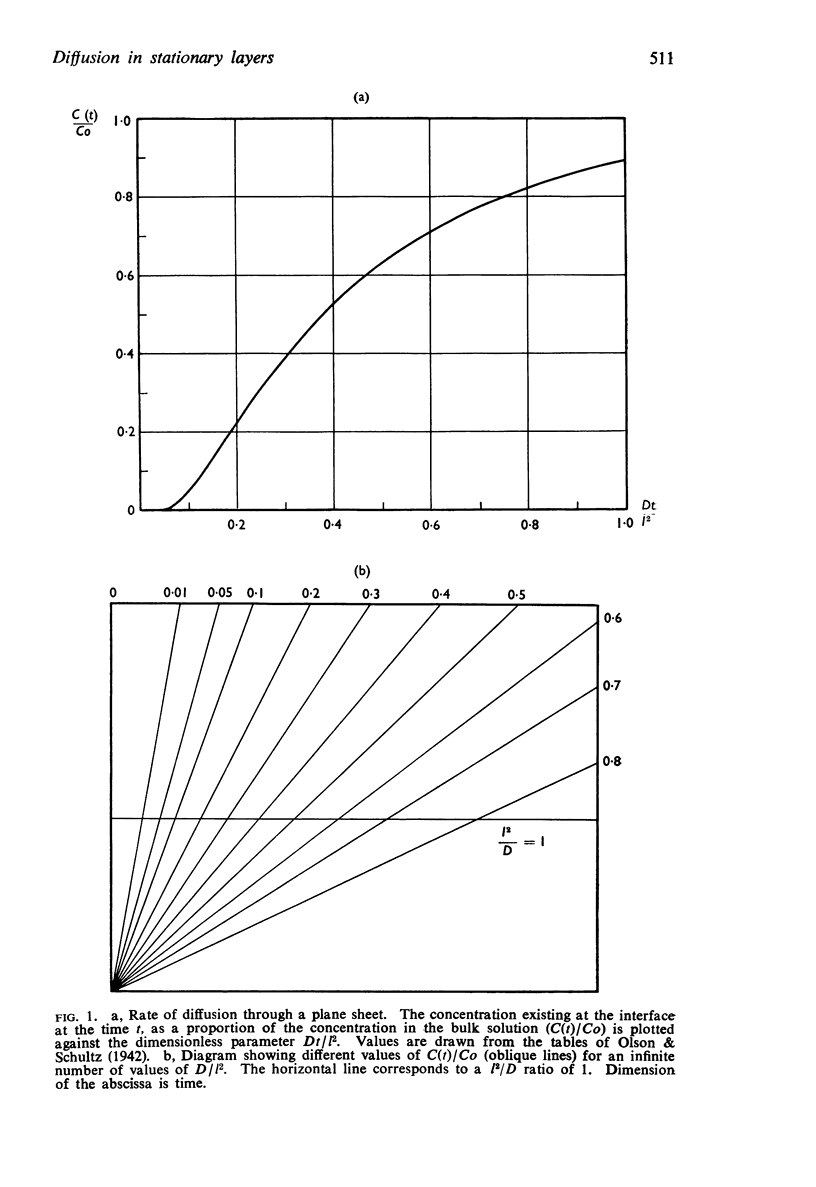
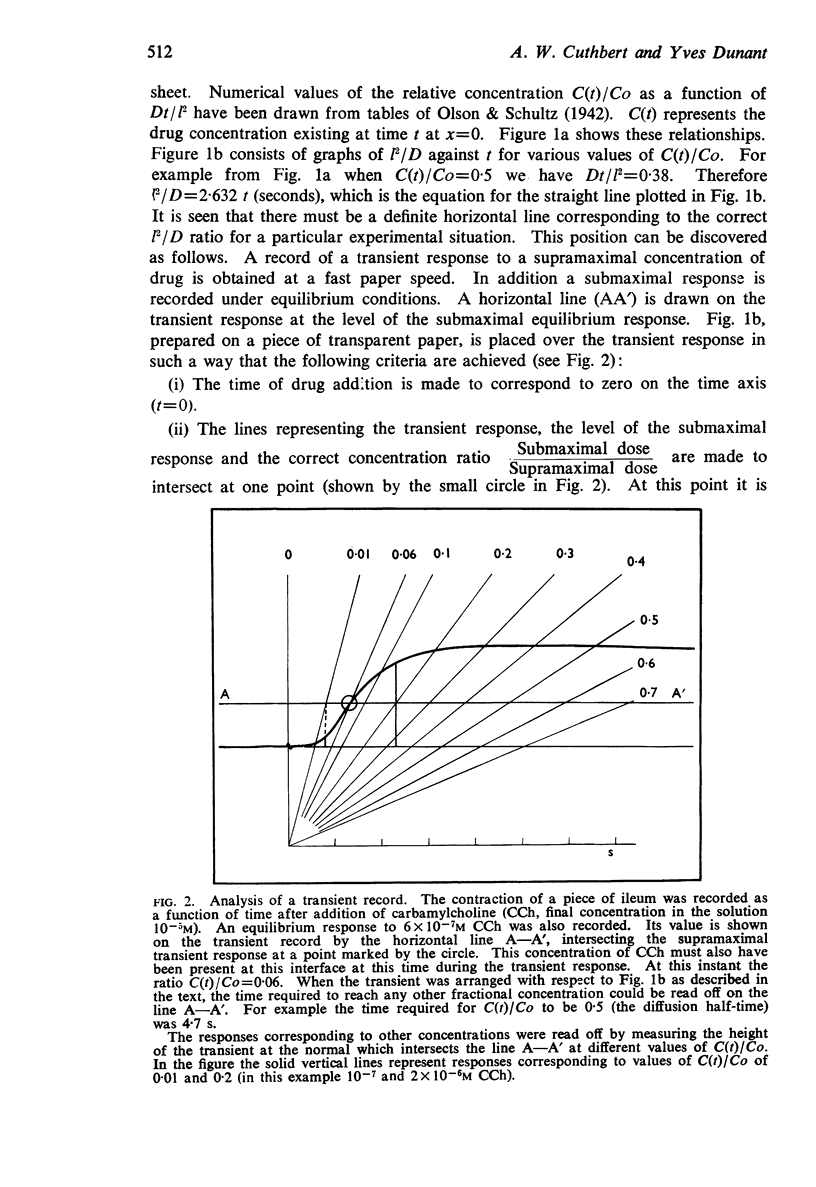
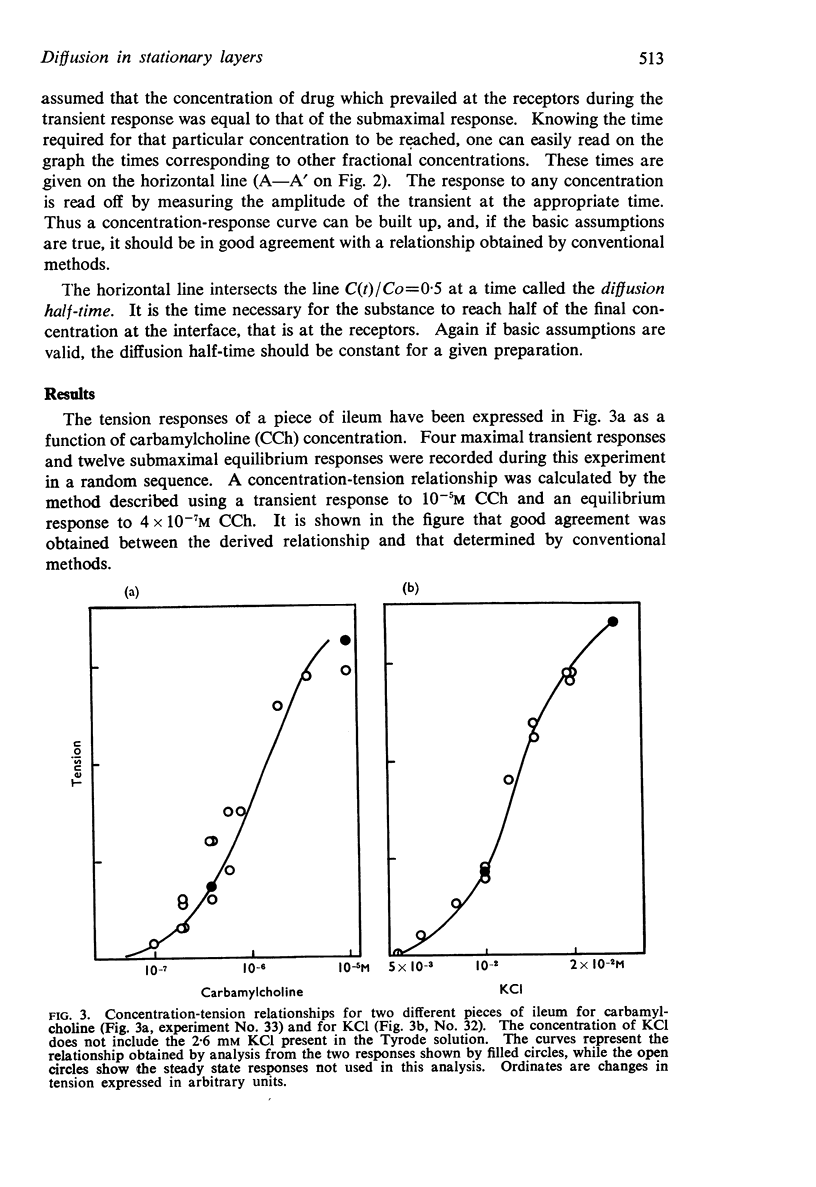
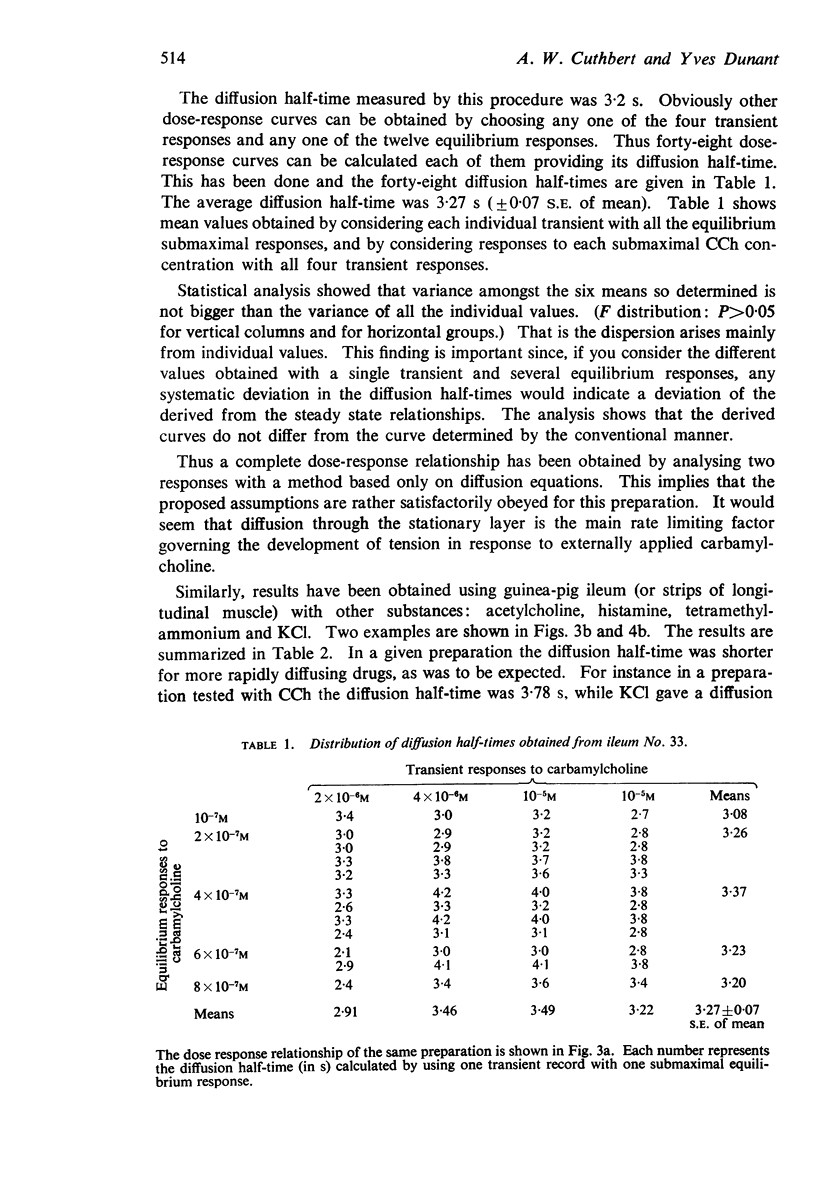
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgen A. S. The drug-receptor complex. J Pharm Pharmacol. 1966 Mar;18(3):137–149. doi: 10.1111/j.2042-7158.1966.tb07840.x. [DOI] [PubMed] [Google Scholar]
- Cook R. P. The antagonism of acetyl choline by methylene blue. J Physiol. 1926 Dec 10;62(2):160–165. doi: 10.1113/jphysiol.1926.sp002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Local activity at a depolarized nerve-muscle junction. J Physiol. 1955 May 27;128(2):396–411. doi: 10.1113/jphysiol.1955.sp005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty J., House C. R. Unstirred layers in frog skin. J Physiol. 1966 Jan;182(1):66–78. doi: 10.1113/jphysiol.1966.sp007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y. Organisation topographique et fonctionnelle du ganglion cervical supérieur chez le Rat. J Physiol (Paris) 1967 Jan-Feb;59(1):17–38. [PubMed] [Google Scholar]
- GINZBURG B. Z., KATCHALSKY A. THE FRICTIONAL COEFFICIENTS OF THE FLOWS OF NON-ELECTROLYTES THROUGH ARTIFICIAL MEMBRANES. J Gen Physiol. 1963 Nov;47:403–418. doi: 10.1085/jgp.47.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The mode of action of nicotine and curari, determined by the form of the contraction curve and the method of temperature coefficients. J Physiol. 1909 Dec 23;39(5):361–373. doi: 10.1113/jphysiol.1909.sp001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Miller J. W., Lewis J. E. Drugs affecting smooth muscle. Annu Rev Pharmacol. 1969;9:147–172. doi: 10.1146/annurev.pa.09.040169.001051. [DOI] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- PATON W. D., WAUD D. R. A QUANTITATIVE INVESTIGATION OF THE RELATIONSHIP BETWEEN RATE OF ACCESS OF A DRUG TO RECEPTOR AND THE RATE OF ONSET OR OFFSET OF ACTION. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 May 11;248:124–143. doi: 10.1007/BF00246668. [DOI] [PubMed] [Google Scholar]


