Abstract
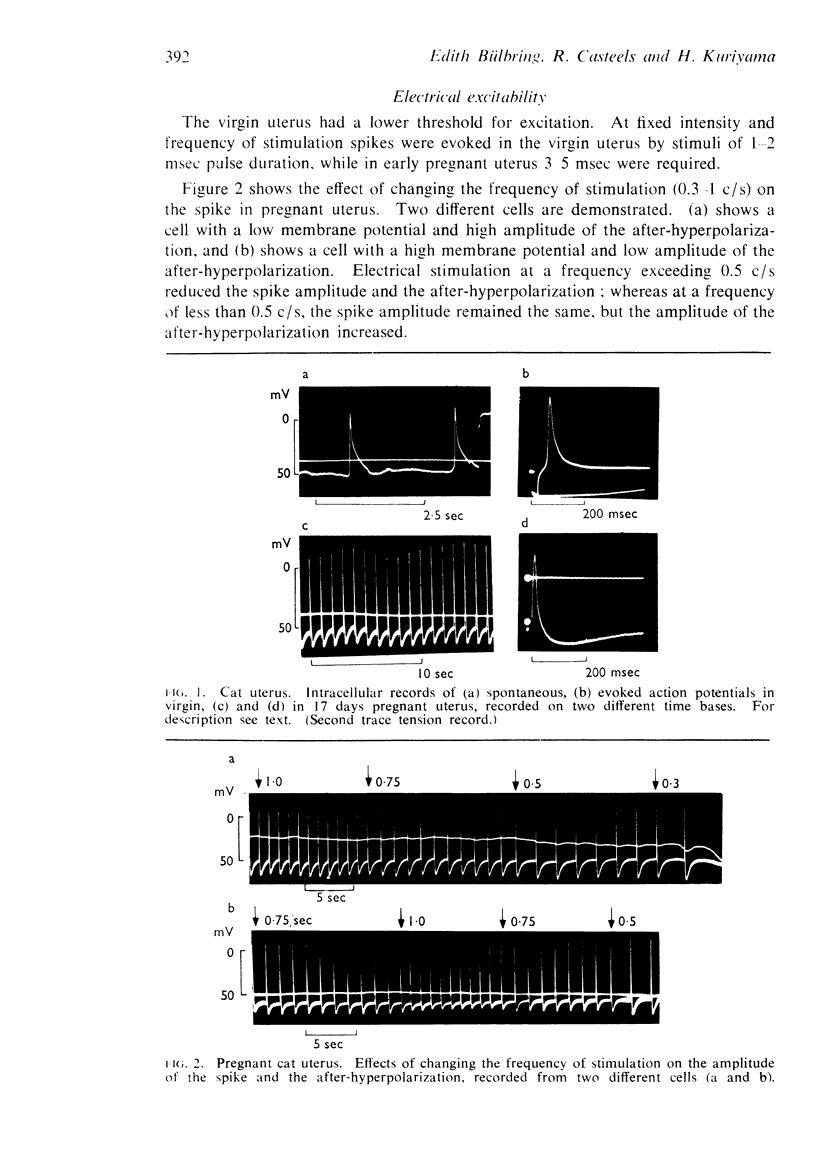
1. Cats, virgin and 17 days pregnant, and guinea-pigs, virgin and 14-60 days pregnant, or treated for 1-8 days with oestradiol+progesterone, were used. The response of the uterus to adrenaline and noradrenaline was observed and, in pieces from the same tissues, the resting and active membrane potentials were recorded and the ionic content was determined.
2. Adrenaline and noradrenaline relaxed the virgin cat uterus, adrenaline being 20-100 times more potent in vivo and about 10 times or less in vitro.
3. Adrenaline and noradrenaline caused contraction of the early pregnant cat uterus, the ratio of potency being about 1.
4. Adrenaline and noradrenaline had a biphasic effect on the guinea-pig uterus in all conditions. The ratio of potency was about 1.
5. The mean membrane potential was 48 mV in virgin cat uterus and 64 mV on the seventeenth day of pregnancy.
6. In guinea-pigs the average membrane potential increased from 38 mV in the virgin uterus to 58 mV on the thirtieth day of pregnancy. A similar increase was produced by eight daily injections of 5 μg oestradiol and, on the last 4 days, additional 1.5 mg progesterone.
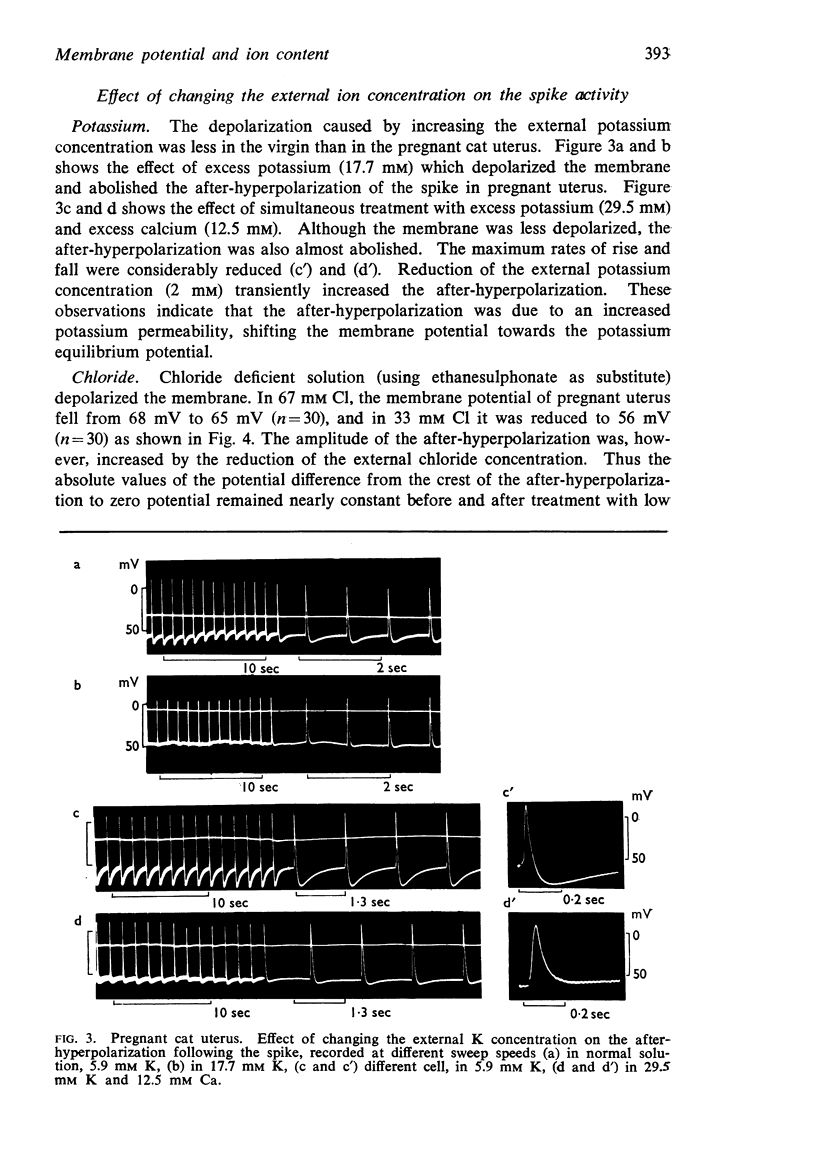
7. In the cat, no significant change in K and Na content was observed during pregnancy. The intracellular chloride content, however, rose from 51.5 m-moles/1. fibre water in the virgin uterus to 89 m-moles in the early pregnant uterus. As a result, the calculated chloride equilibrium potential changed from - 25 mV in virgin uterus to - 11 mV in pregnant uterus.
8. In the guinea-pig no significant change in ion content was observed and the calculated potassium and chloride equilibrium potentials remained both unaltered during pregnancy.
9. In contrast to guinea-pig uterus in all conditions, and to virgin cat uterus, early pregnant cat uterus was not spontaneously active and excess calcium caused no hyperpolarization.
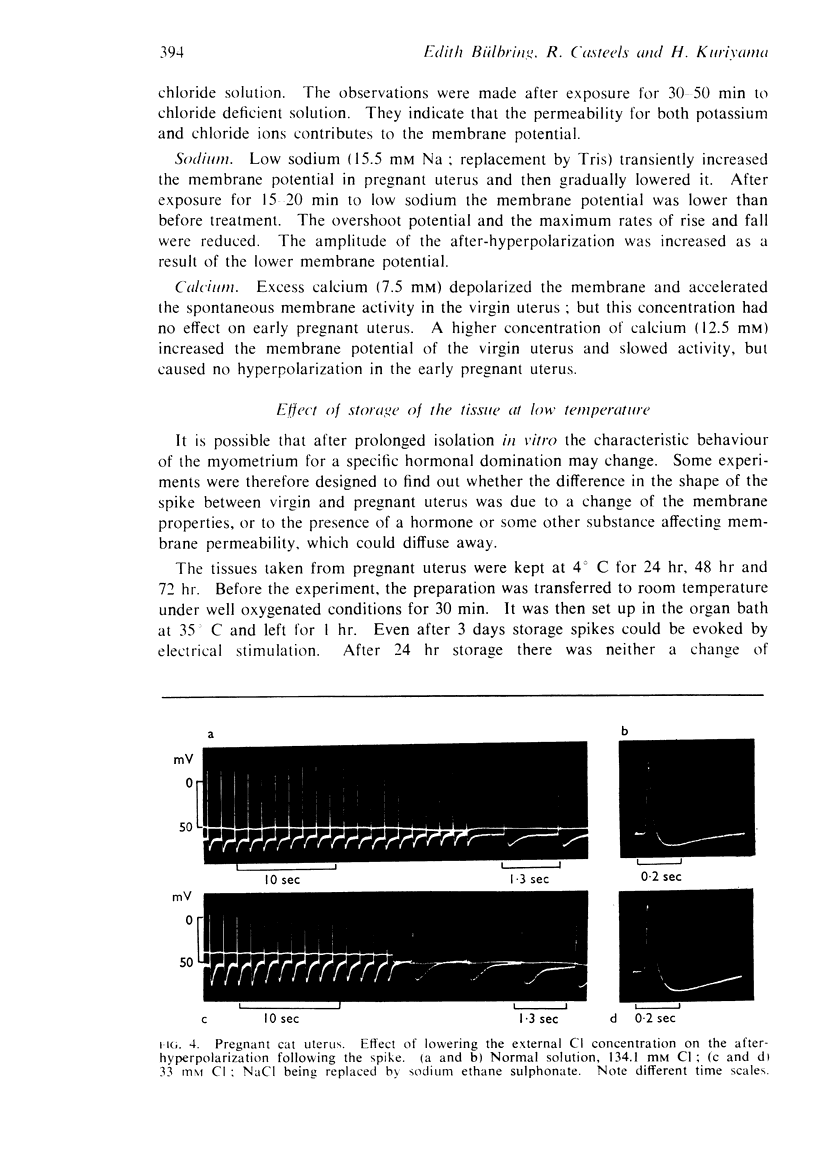
10. The reversal of the uterine response to adrenaline as a result of pregnancy is discussed in relation to the increase of the intracellular chloride content which was only observed in the cat.
Full text
PDF



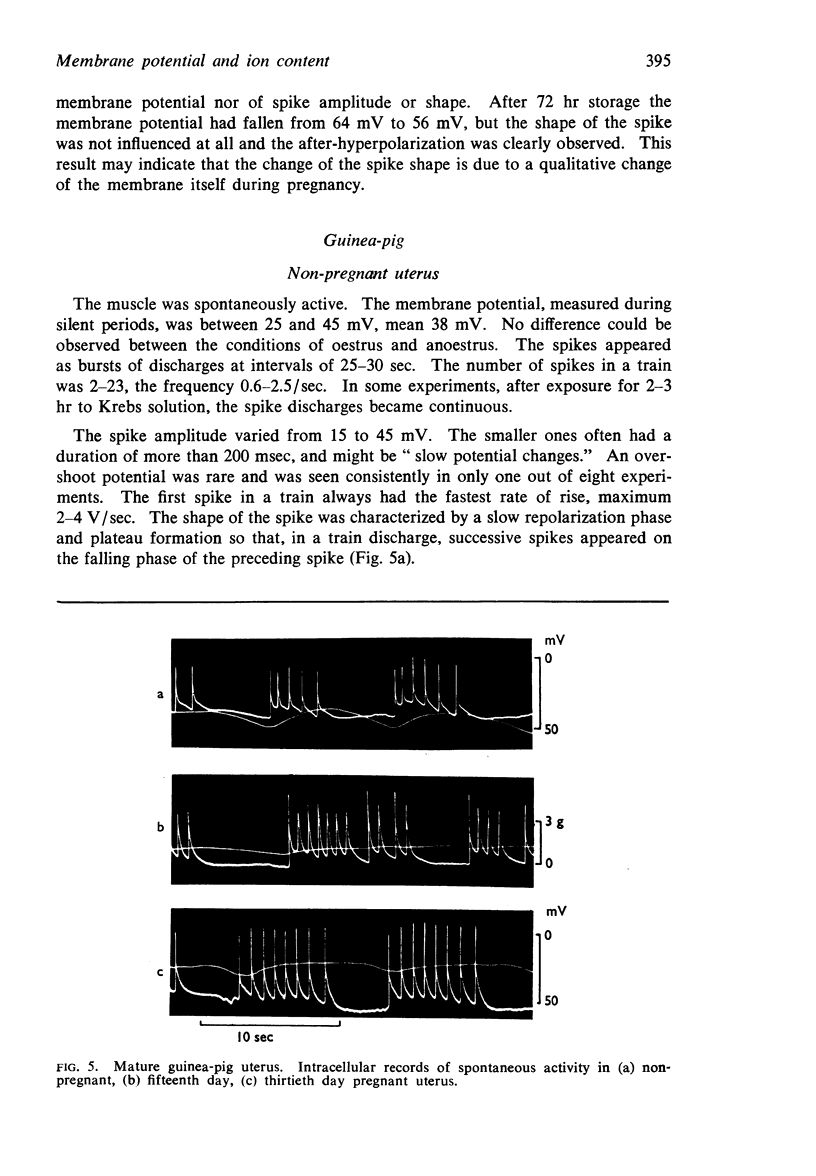

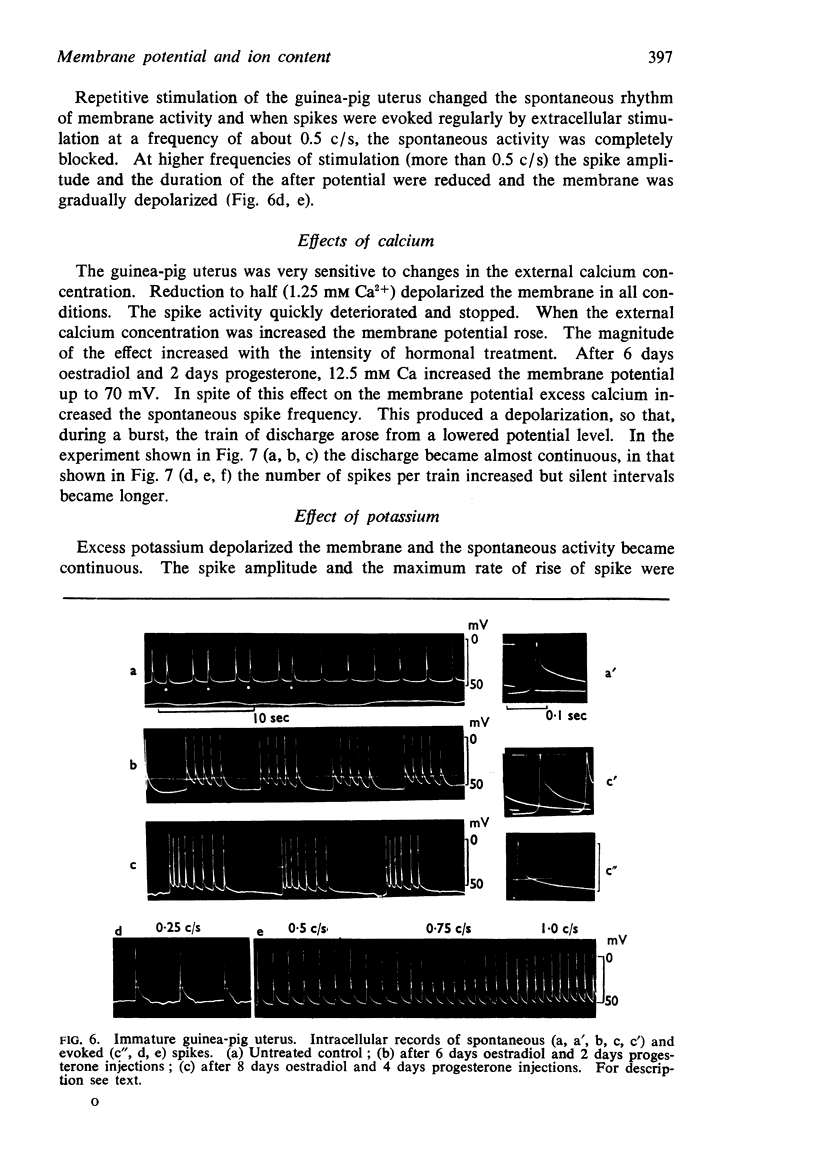
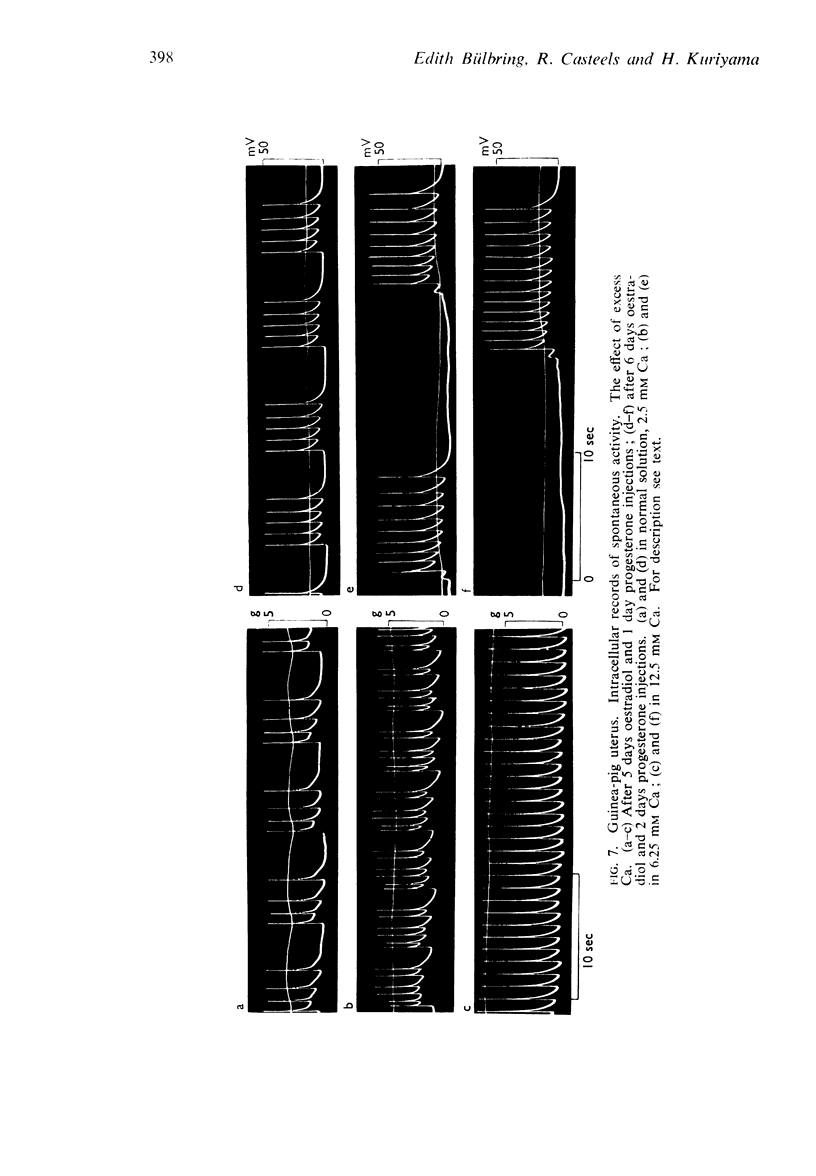
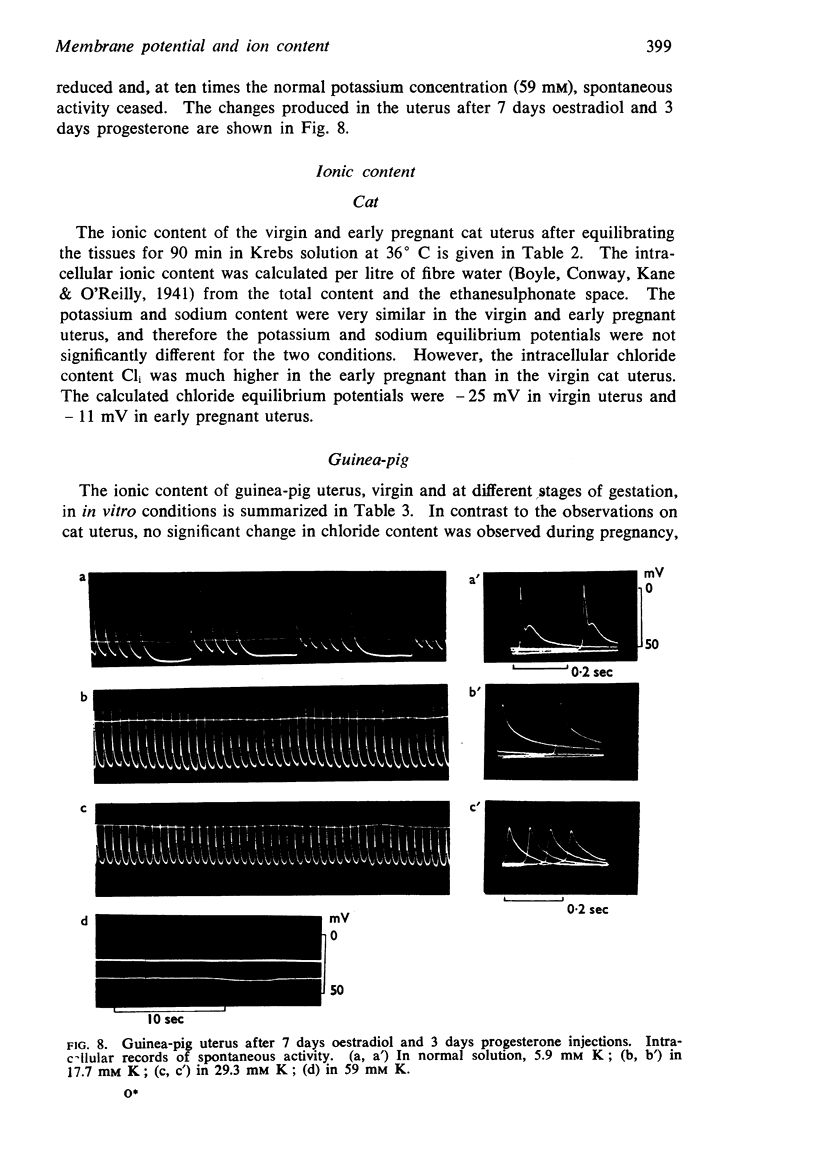
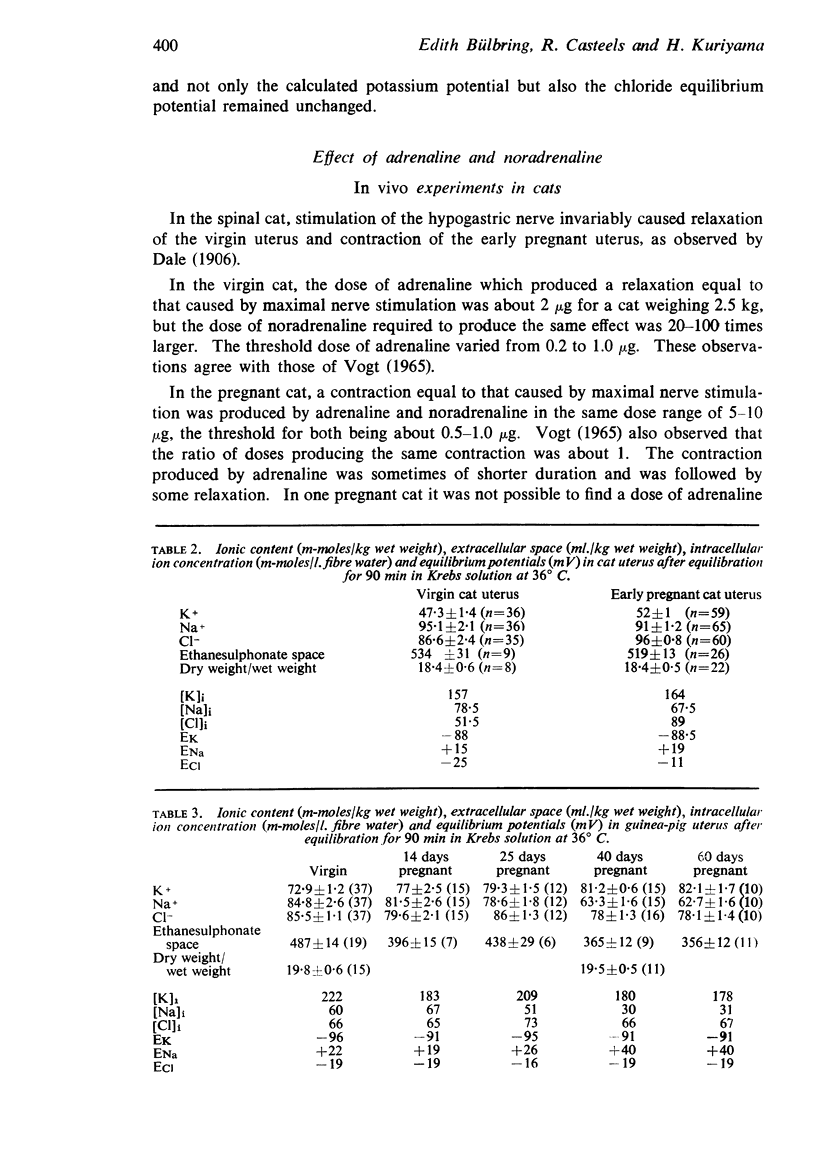
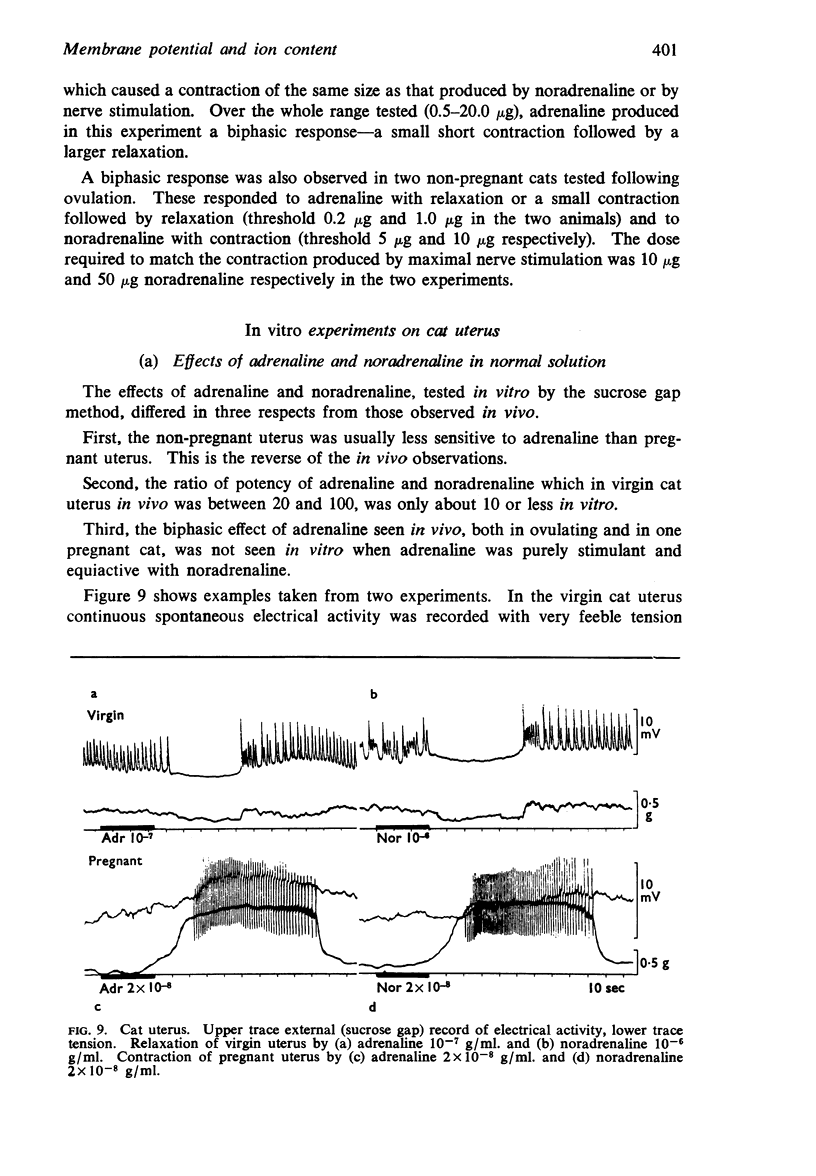
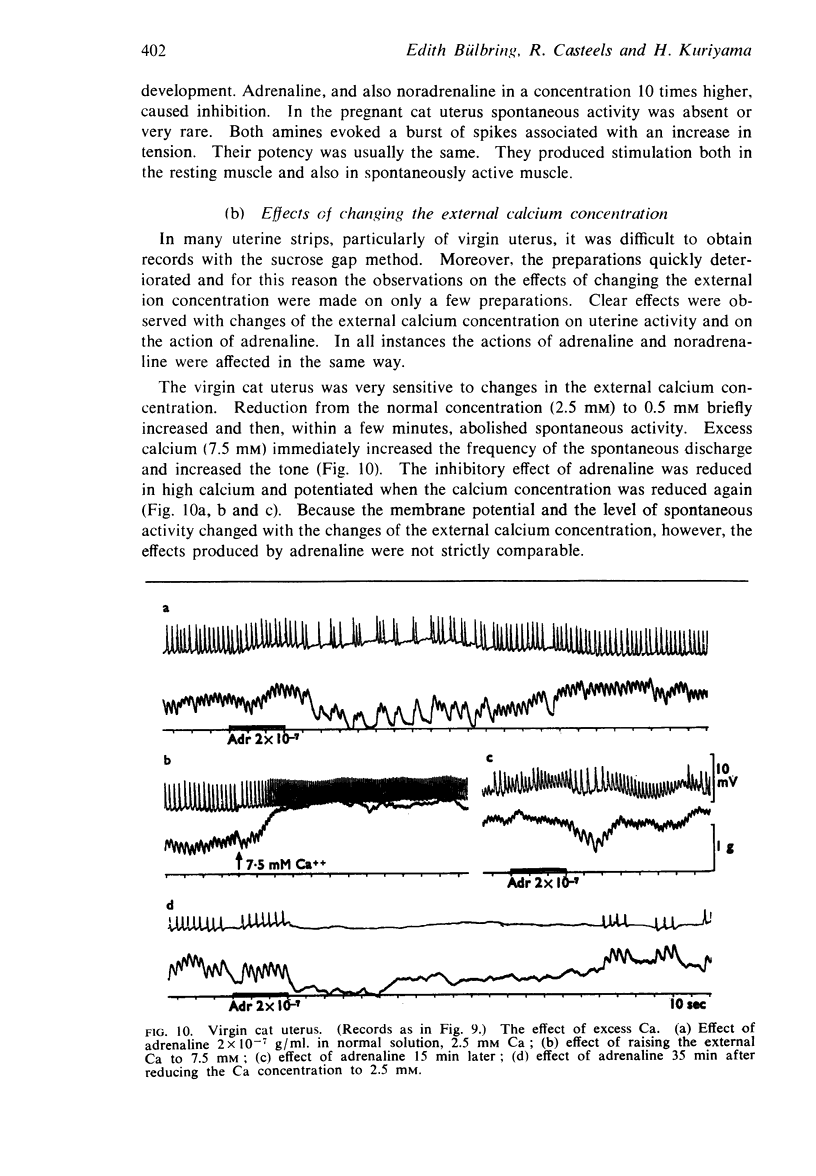
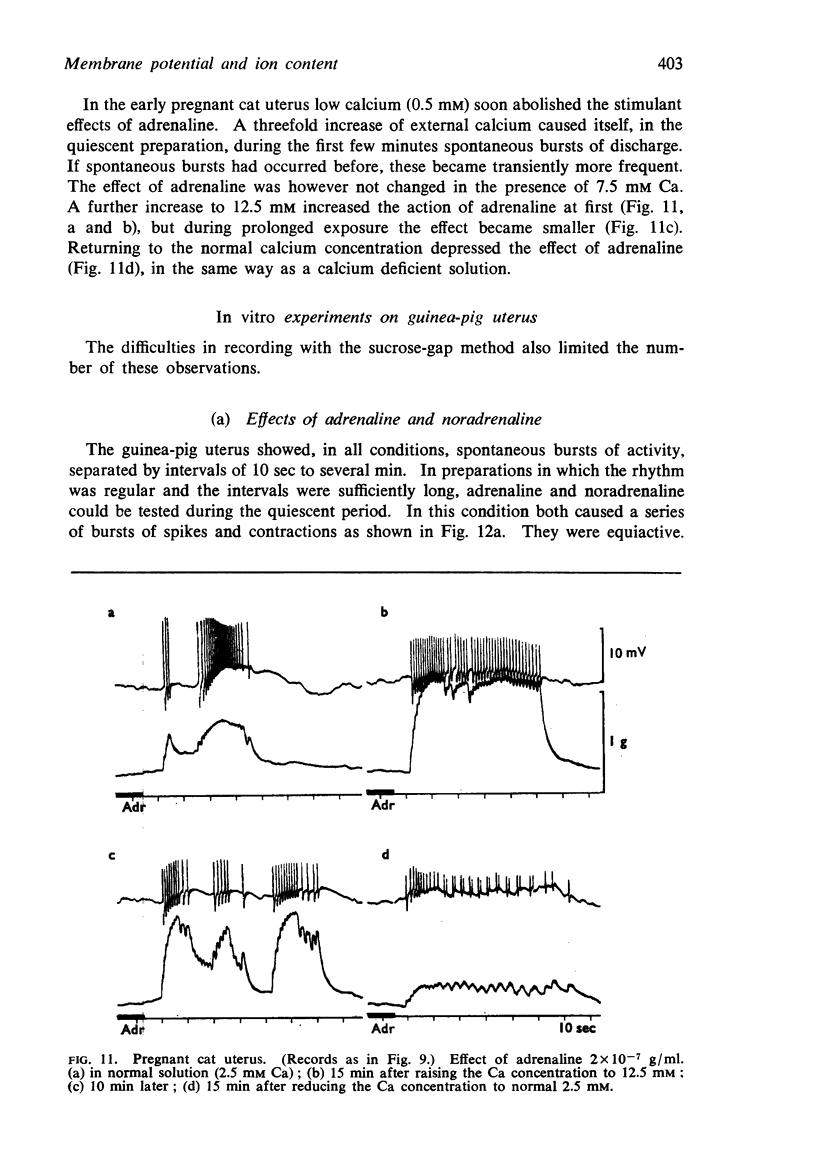
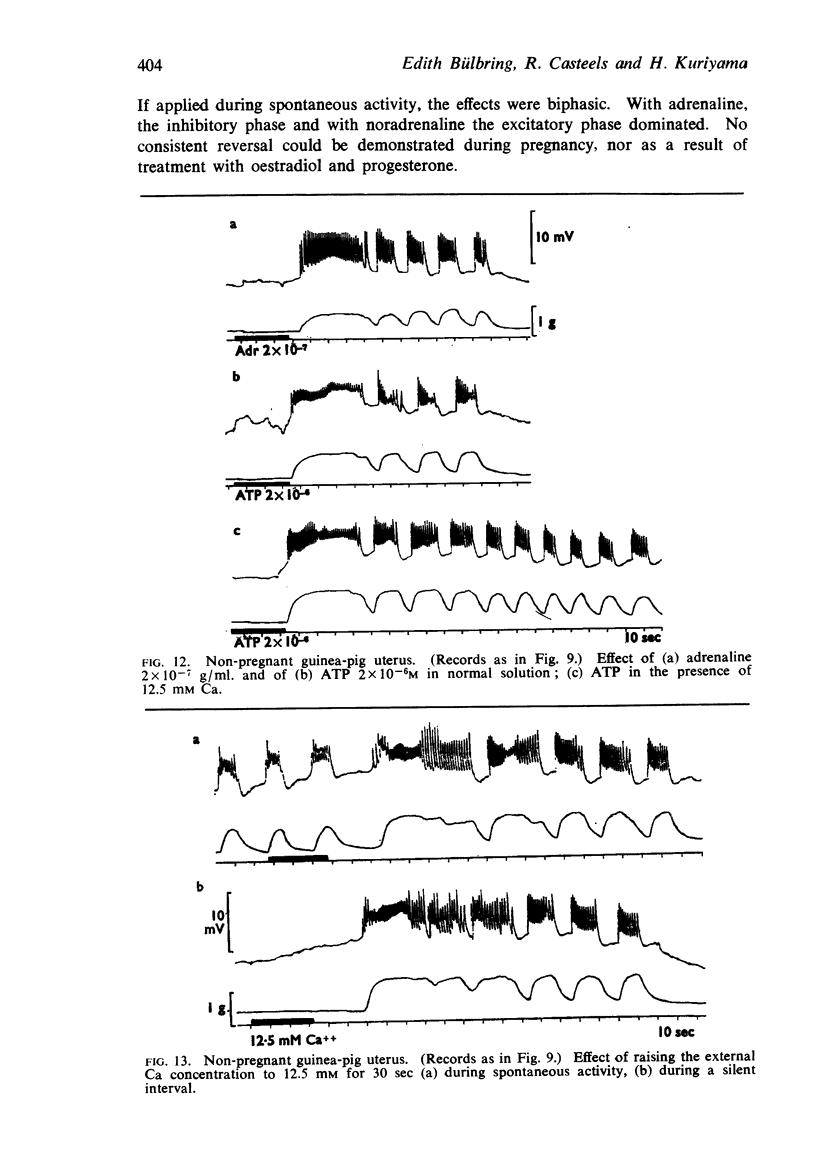



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J., Kane F., O'reilly H. L. Volume of interfibre spaces in frog muscle and the calculation of concentrations in the fibre water. J Physiol. 1941 Jun 30;99(4):401–414. doi: 10.1113/jphysiol.1941.sp003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueding E., Bülbring E., Gercken G., Hawkins J. T., Kuriyama H. The effect of adrenaline on the adenosine otriphosphate and creatine phosphate content of intestinal smooth muscle. J Physiol. 1967 Nov;193(1):187–212. doi: 10.1113/jphysiol.1967.sp008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kuriyama H. Membrane potential and ion content in the smooth muscle of the guinea-pig's taenia coli at different external potassium concentrations. J Physiol. 1966 May;184(1):120–130. doi: 10.1113/jphysiol.1966.sp007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Phénomènes osmotiques et répartitions des ions dans le muscle lisse du taenia coli du cobaye. J Physiol (Paris) 1965 Sep-Oct;57(5):581–581. [PubMed] [Google Scholar]
- Dale H. H. On some physiological actions of ergot. J Physiol. 1906 May 31;34(3):163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMANSEN K. The effect of adrenaline, noradrenaline and isoprenaline on the guinea-pig uterus. Br J Pharmacol Chemother. 1961 Feb;16:116–128. doi: 10.1111/j.1476-5381.1961.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSEN V. K., LEVI H., USSING H. H. The mode of passage of chloride ions through the isolated frog skin. Acta Physiol Scand. 1952 Jun 6;25(2-3):150–163. doi: 10.1111/j.1748-1716.1952.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Morton I. K. The effect of noradrenaline on the permeability of depolarized intestinal smooth muscle to inorganic ions. J Physiol. 1967 Feb;188(3):373–386. doi: 10.1113/jphysiol.1967.sp008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H., CSAPO A. A study of the parturient uterus with the microelectrode technique. Endocrinology. 1961 Jun;68:1010–1025. doi: 10.1210/endo-68-6-1010. [DOI] [PubMed] [Google Scholar]
- MARSHALL J. M. Regulation of activity in uterine smooth muscle. Physiol Rev Suppl. 1962 Jul;5:213–227. [PubMed] [Google Scholar]
- Miller J. W. Adrenergic receptors in the myometrium. Ann N Y Acad Sci. 1967 Feb 10;139(3):788–798. doi: 10.1111/j.1749-6632.1967.tb41247.x. [DOI] [PubMed] [Google Scholar]
- THIERSCH J. B., LANDA J. F., WEST T. C. Transmembrane potentials in the rat myometrium during pregnancy. Am J Physiol. 1959 Apr;196(4):901–904. doi: 10.1152/ajplegacy.1959.196.4.901. [DOI] [PubMed] [Google Scholar]
- Vogt M. Transmitter released in the cat uterus by stimulation of the hypogastric nerves. J Physiol. 1965 Jul;179(1):163–171. doi: 10.1113/jphysiol.1965.sp007655. [DOI] [PMC free article] [PubMed] [Google Scholar]


