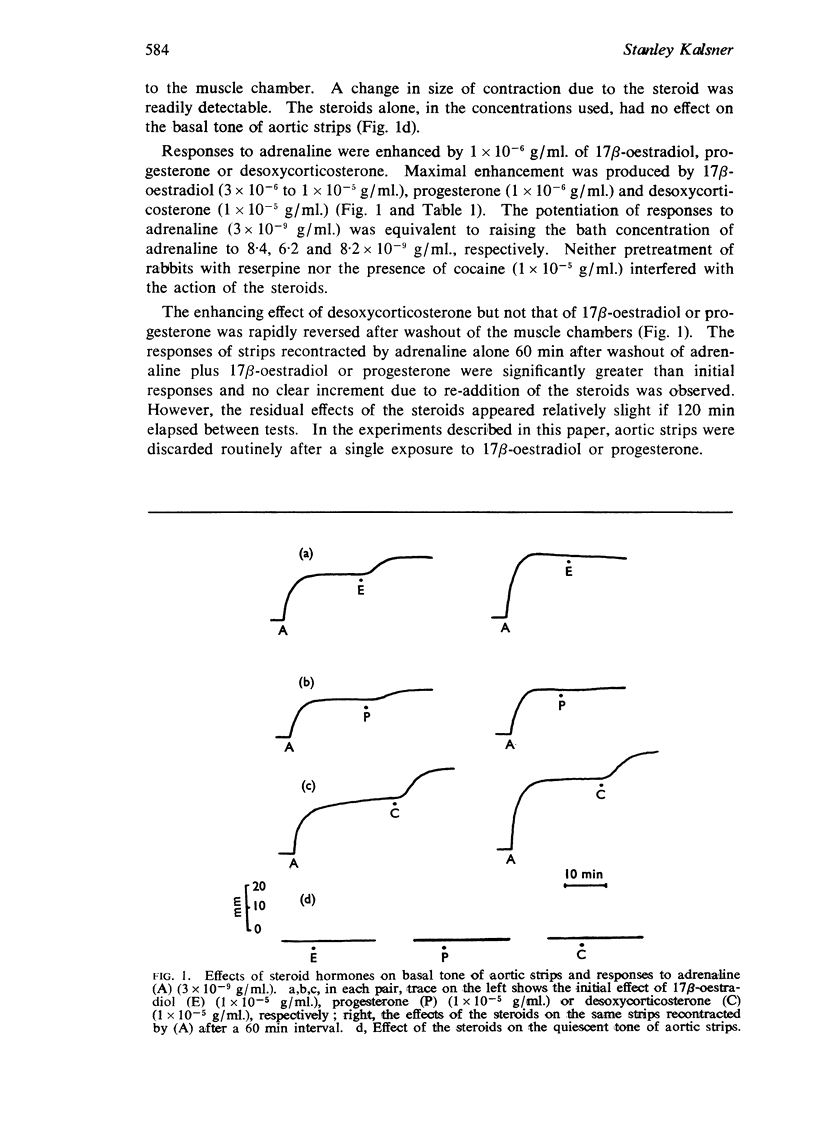
Abstract
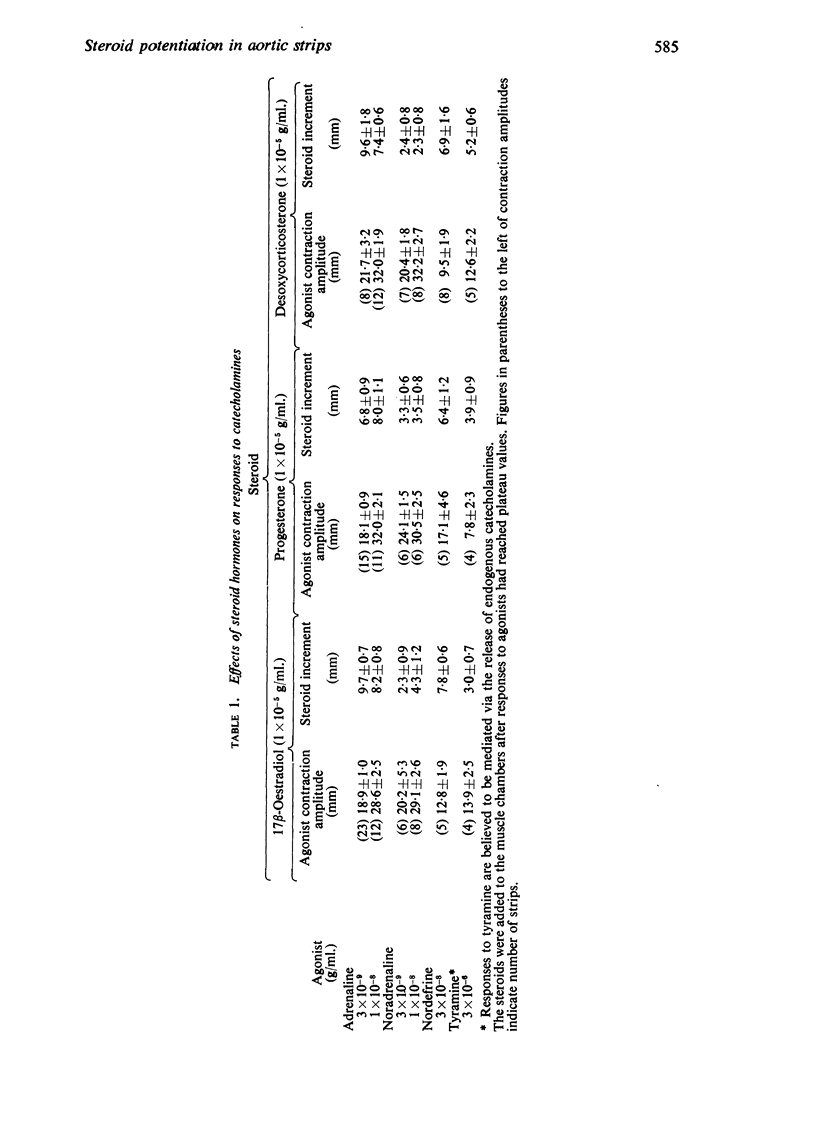
1. Responses to catecholamines (adrenaline, noradrenaline, nordefrine) were enhanced by 17β-oestradiol, progesterone and desoxycorticosterone in untreated and reserpine pretreated aortic strips. Responses to tyramine, believed mediated via endogenous catecholamines, were enhanced only in untreated strips.
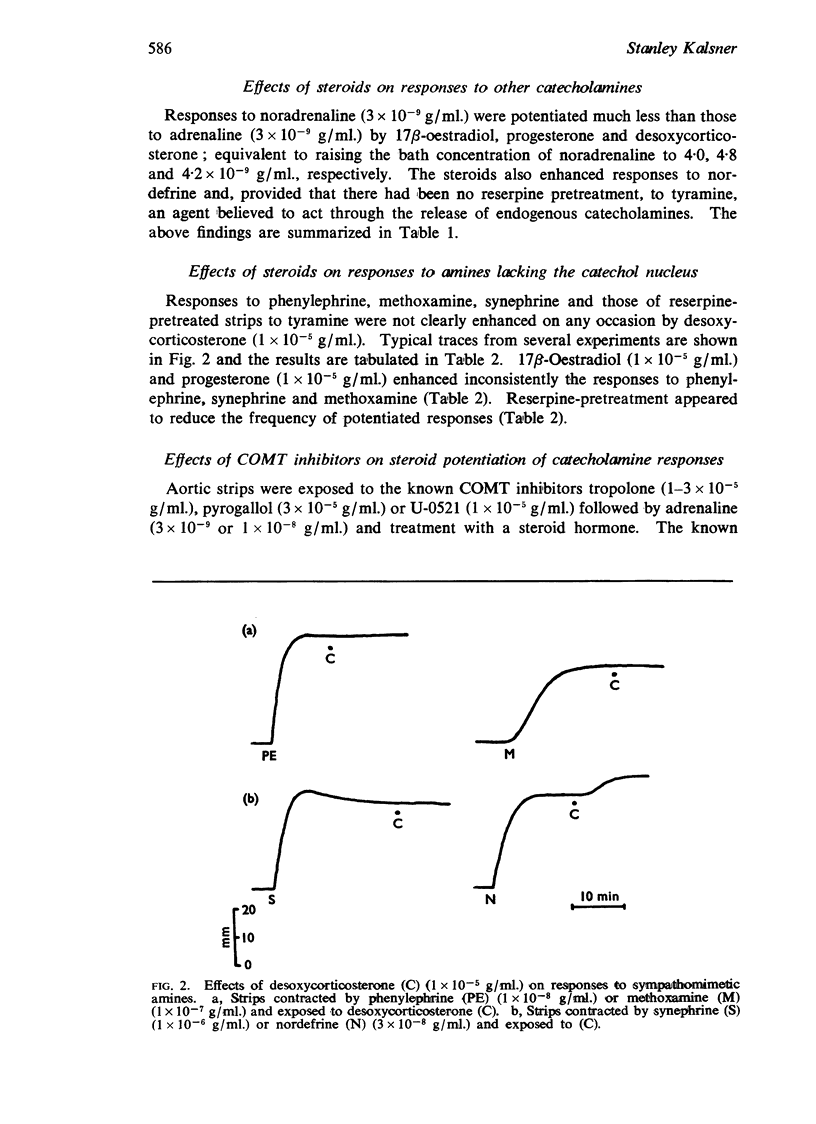
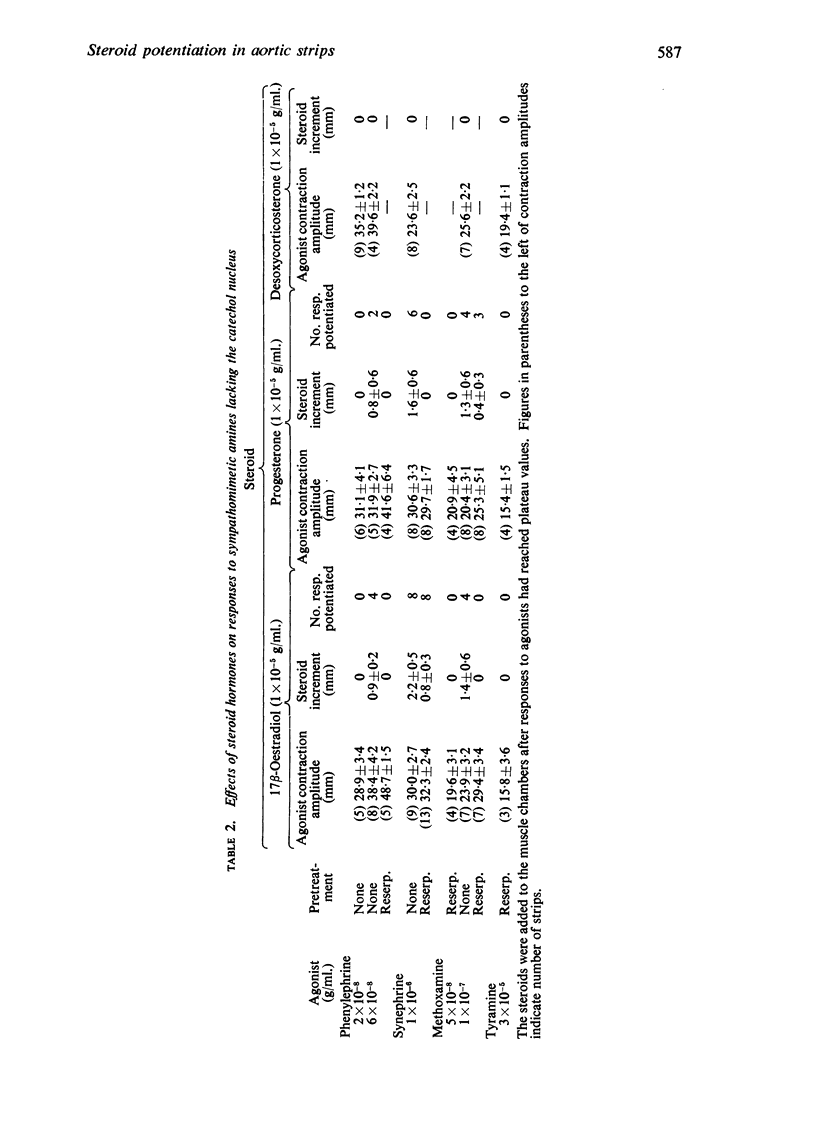
2. Responses to sympathomimetic amines lacking the catechol nucleus (phenylephrine, synephrine, methoxamine) were potentiated inconsistently by the steroids and reserpine pretreatment reduced markedly the frequency of potentiated responses.
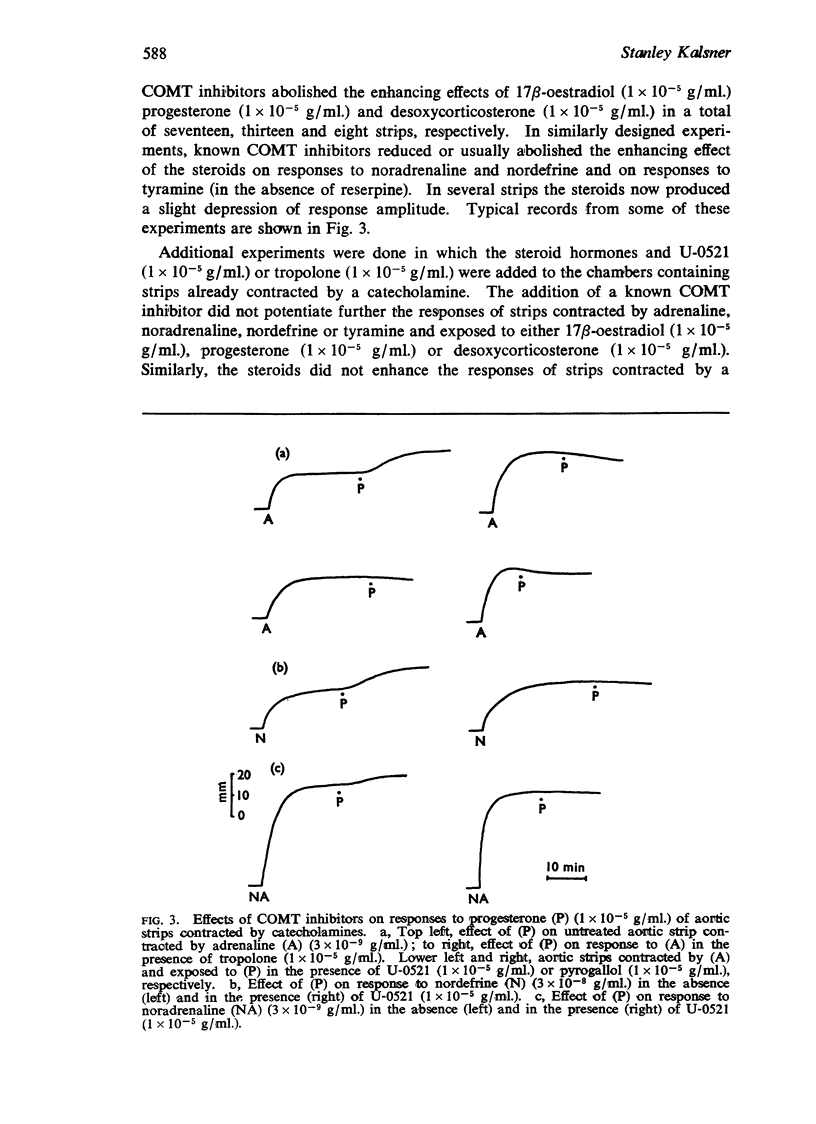
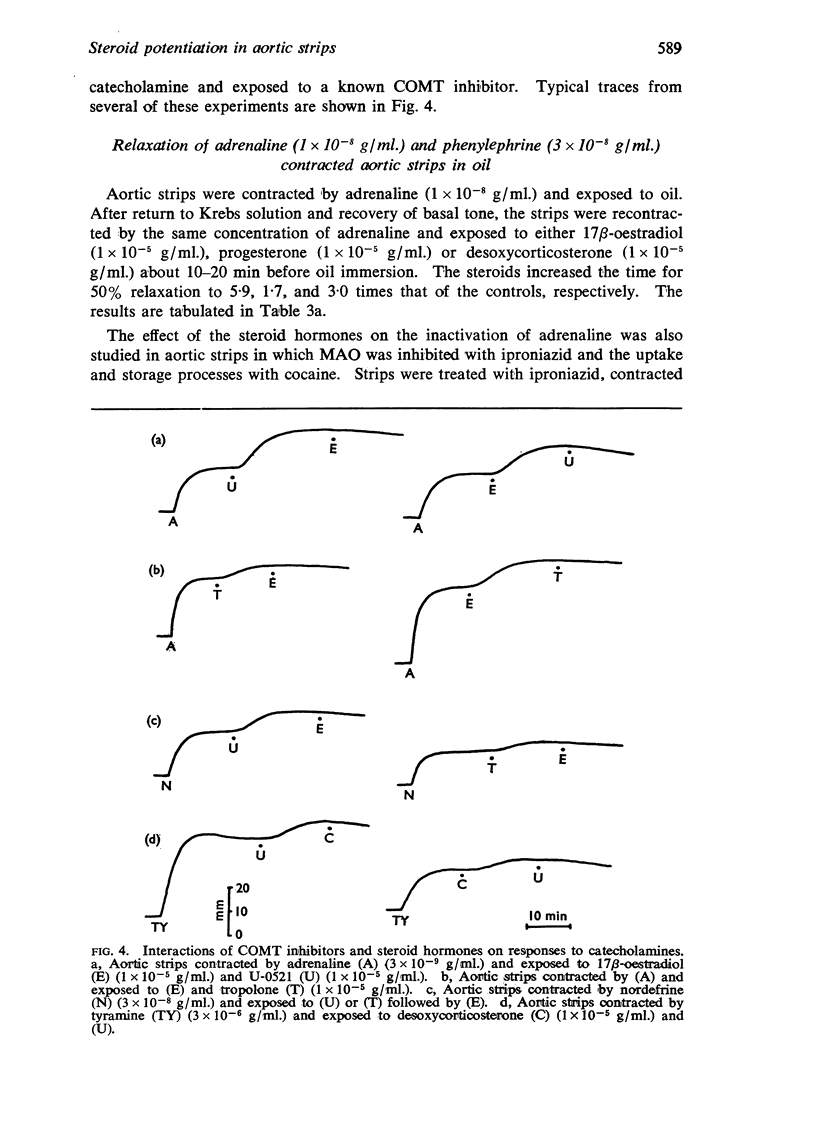
3. Known inhibitors of catechol-O-methyl transferase (tropolone, U-0521, pyrogallol) potentiated responses to catecholamines and abolished the enhancing effects of the steroids—when the steroids were given first, there was no further increase in response to catecholamines on adding inhibitors of catechol-O-methyl transferase.
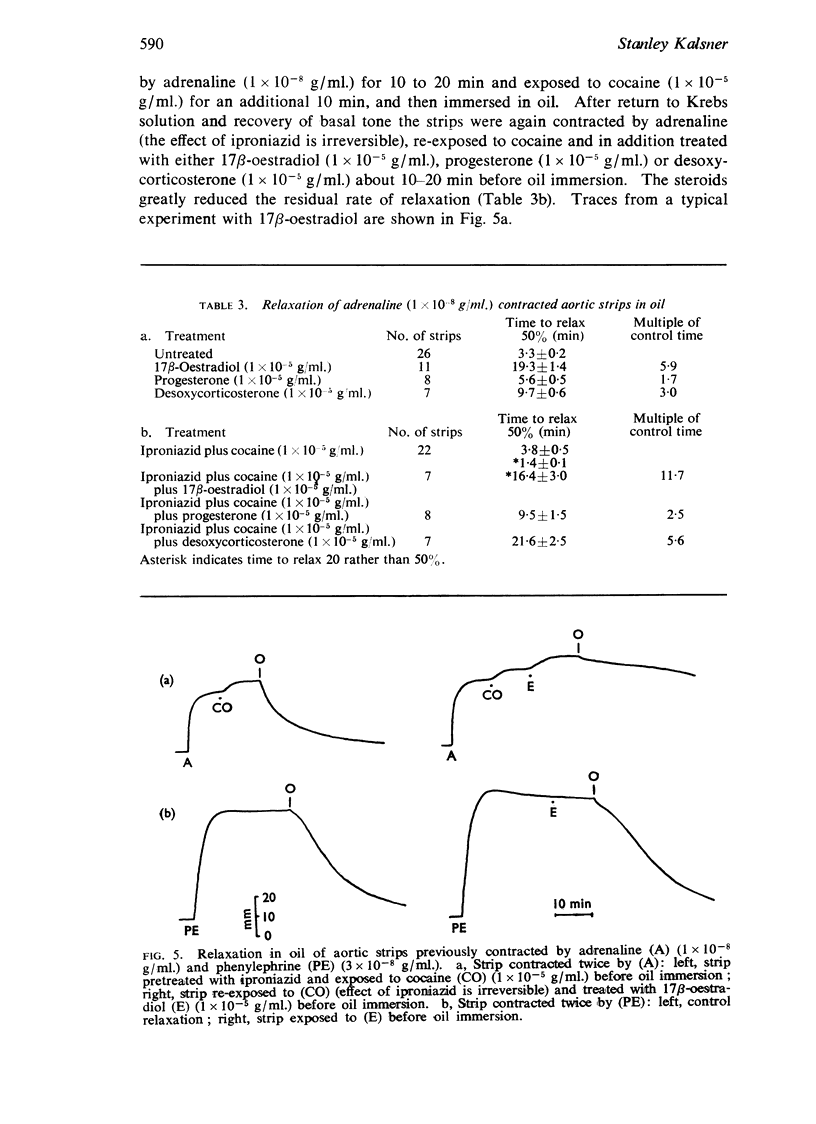
4. Experiments with the oil-immersion technique, to eliminate diffusion of drug from the tissue, indicated that 17β-oestradiol, progesterone and desoxycorticosterone decreased the rate at which aortic strips inactivated adrenaline by O-methylation.
5. It is concluded that 17β-oestradiol, progesterone and desoxycorticosterone potentiate responses to catecholamines in aortic strips by inhibiting a major mechanism for their inactivation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., WEIL-MALHERBE H., TOMCHICK R. The physiological disposition of H3-epinephrine and its metabolite metanephrine. J Pharmacol Exp Ther. 1959 Dec;127:251–256. [PubMed] [Google Scholar]
- BELLEAU B., BURBA J. Tropolones: a unique class of potent non-competitive inhibitors of S-adenosylmethionine-catechol methyltransferase. Biochim Biophys Acta. 1961 Nov 25;54:195–196. doi: 10.1016/0006-3002(61)90956-8. [DOI] [PubMed] [Google Scholar]
- BOHR D. F., BRODIE D. C., CHEU D. H. Effect of electrolytes on arterial muscle contraction. Circulation. 1958 Apr;17(4 Pt 2):746–749. doi: 10.1161/01.cir.17.4.746. [DOI] [PubMed] [Google Scholar]
- CHA K. S., LEE W. C., RUDZIK A., MILLER J. W. A COMPARISON OF THE CATECHOLAMINE CONCENTRATIONS OF UTERI FROM SEVERAL SPECIES AND THE ALTERATIONS WHICH OCCUR DURING PREGNANCY. J Pharmacol Exp Ther. 1965 Apr;148:9–13. [PubMed] [Google Scholar]
- Eisenfeld A. J., Krakoff L., Iversen L. L., Axelrod J. Inhibition of the extraneuronal metabolism of noradrenaline in the isolated heart by adrenergic blocking agents. Nature. 1967 Jan 21;213(5073):297–298. doi: 10.1038/213297a0. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R. F. The pharmacology of vascular smooth muscle. Pharmacol Rev. 1955 Jun;7(2):183–265. [PubMed] [Google Scholar]
- Giles R. E., Miller J. W. The catechol-O-methyl transferase activity and endogenous catecholamine content of various tissues in the rat and the effect of administration of U-0521 (3',4'-dihydroxy-2-methyl propiophenone). J Pharmacol Exp Ther. 1967 Nov;158(2):189–194. [PubMed] [Google Scholar]
- Green R. D., 3rd, Miller J. W. Catecholamine Concentrations: Changes in Plasma of Rats during Estrous Cycle and Pregnancy. Science. 1966 Feb 18;151(3712):825–826. doi: 10.1126/science.151.3712.825. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Mechanism of hydrocortisone potentiation of responses to epinephrine and norepinephrine in rabbit aorta. Circ Res. 1969 Mar;24(3):383–395. doi: 10.1161/01.res.24.3.383. [DOI] [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. A method for the study of mechanisms of drug disposition in smooth muscle. Can J Physiol Pharmacol. 1968 Sep;46(5):719–730. doi: 10.1139/y68-113. [DOI] [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Disposition of norepinephrine and epinephrine in vascular tissue, determined by the technique of oil immersion. J Pharmacol Exp Ther. 1969 Feb;165(2):152–165. [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Disposition of phenylephrine in vascular tissue, determined by the oil-immersion technique. J Pharmacol Exp Ther. 1968 Sep;163(1):1–10. [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Effects of a haloalkylamine on responses to and disposition of sympathomimetic amines. Br J Pharmacol. 1969 Mar;35(3):440–455. doi: 10.1111/j.1476-5381.1969.tb08285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAVRIDES C., MISSALA K., D'IORIO A. The effect of 4-methyltropolone on the metabolism of adrenaline. Can J Biochem Physiol. 1963 Jul;41:1581–1587. [PubMed] [Google Scholar]
- RUDZIK A. D., MILLER J. W. The effect of altering the catecholamine content of the uterus on the rate of contractions and the sensitivity of the myometrium to relaxin. J Pharmacol Exp Ther. 1962 Oct;138:88–95. [PubMed] [Google Scholar]
- Spratto G. R., Miller J. W. An investigation of the mechanism by which estradiol-17-beta elevates the epinephrine content of the rat uterus. J Pharmacol Exp Ther. 1968 May;161(1):7–13. [PubMed] [Google Scholar]
- WHITBY L. G., AXELROD J., WEIL-MALHERBE H. The fate of H3-norepinephrine in animals. J Pharmacol Exp Ther. 1961 May;132:193–201. [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J., POTTER L. T. THE DISPOSITION OF CATECHOLAMINES IN THE RAT UTERUS AND THE EFFECT OF DRUGS AND HORMONES. J Pharmacol Exp Ther. 1964 May;144:150–155. [PubMed] [Google Scholar]
- WURTMAN R. J., CHU E. W., AXELROD J. Relation between the oestrous cycle and the binding of catecholamines in the rat uterus. Nature. 1963 May 11;198:547–548. doi: 10.1038/198547a0. [DOI] [PubMed] [Google Scholar]
- ZELLER E. A., BARSKY J. In vivo inhibition of liver and brain monoamine oxidase by 1-Isonicotinyl-2-isopropyl hydrazine. Proc Soc Exp Biol Med. 1952 Nov;81(2):459–461. doi: 10.3181/00379727-81-19910. [DOI] [PubMed] [Google Scholar]


