Abstract
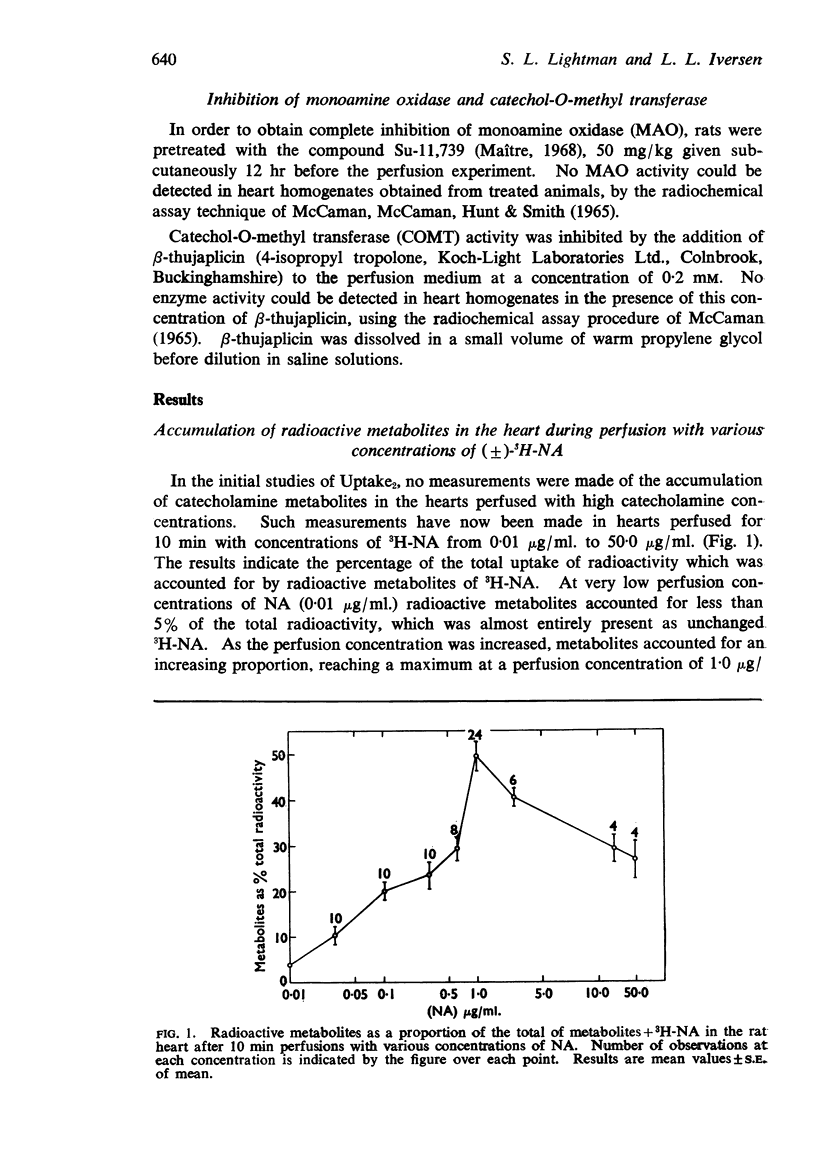
1. (±)-3H-NA and labelled metabolites of NA were estimated in rat hearts after perfusion with various concentrations of NA in the range 0·01-50·0 μg/ml. Labelled metabolites of NA accounted for only a small proportion of the total uptake of radioactivity at low perfusion concentrations, but accounted for 50% of the total uptake at 1 μg NA/ml., thereafter declining to progressively smaller proportions at higher perfusion concentrations.
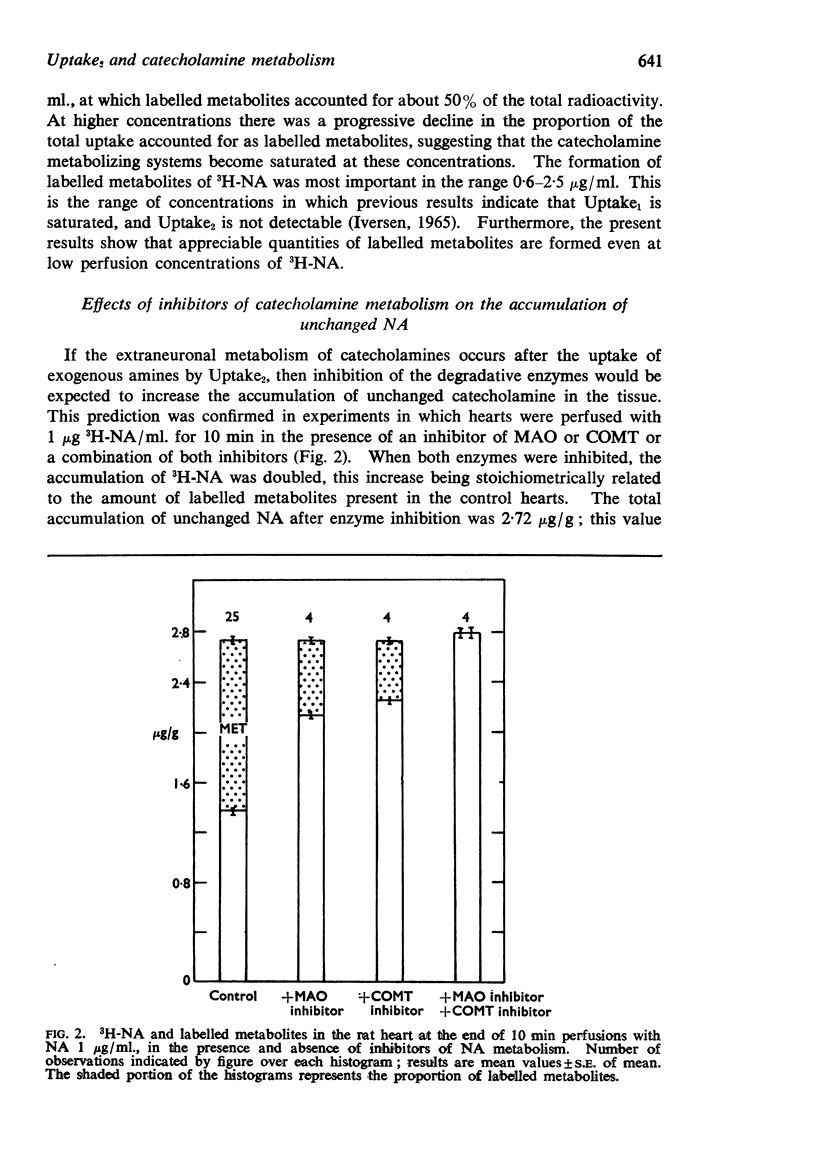
2. If the formation of labelled metabolites of 3H-NA was blocked by a combination of monoamine oxidase and catechol-O-methyl transferase inhibitors, the accumulation of unchanged 3H-NA was doubled when hearts were perfused with 1 μg NA/ml.
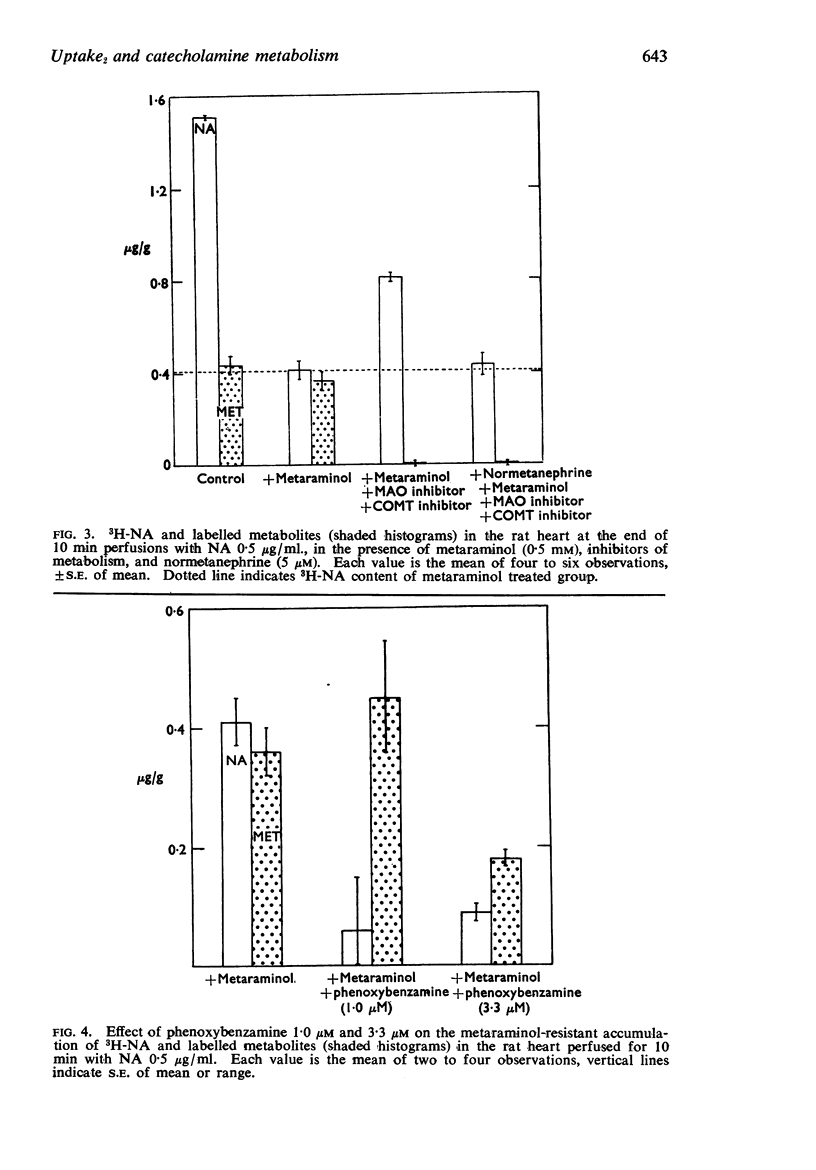
3. In hearts perfused with 0·5 μg NA/ml., an accumulation of unchanged 3H-NA was demonstrated in the presence of a combination of metabolic inhibitors and metaraminol. This appeared to be due to Uptake2, since the accumulation of NA under these conditions could be prevented by a low concentration of normetanephrine.
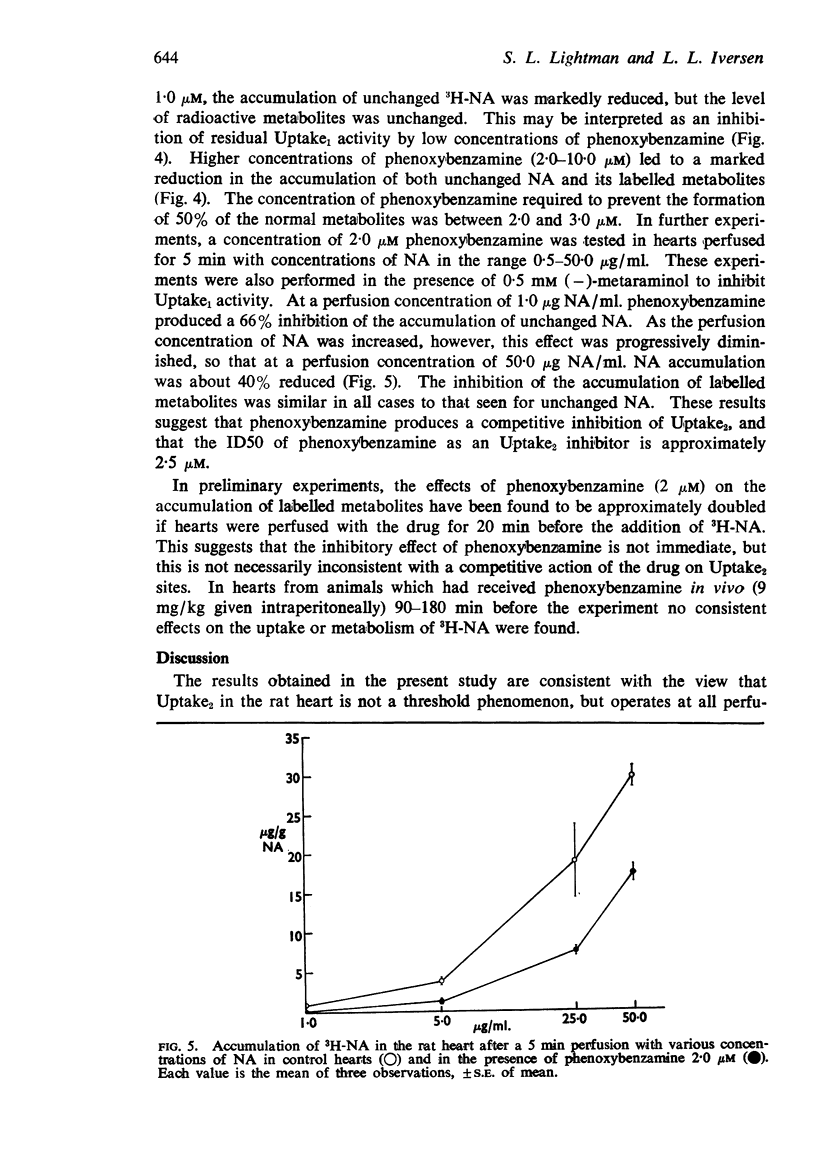
4. Phenoxybenzamine prevented extraneuronal uptake (Uptake2) and metabolism of 3H-NA with an estimated ID50 of 2·5 μM. The inhibition of Uptake2 by phenoxybenzamine (2·0 μM) was diminished at very high NA concentrations, suggesting that the drug may act competitively with NA.
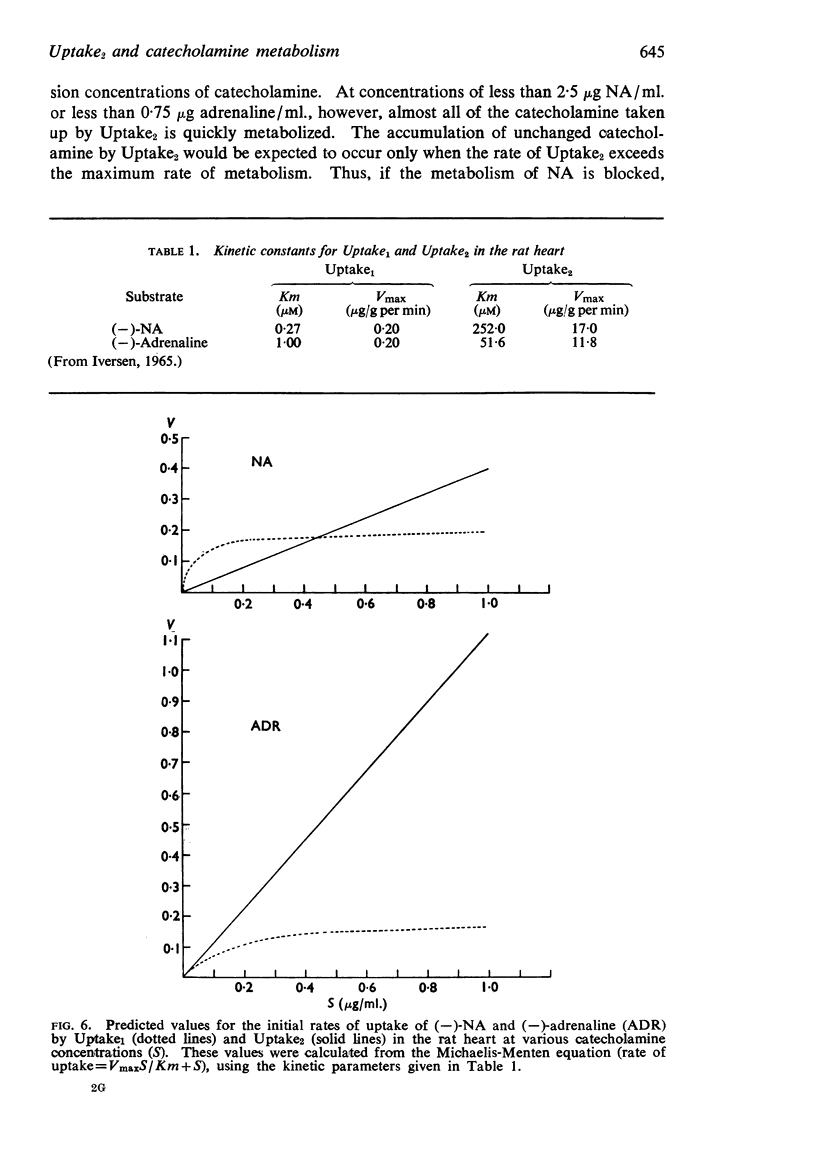
5. It was concluded that Uptake2 operates at all catecholamine concentrations in the rat heart, but that in the lower range (less than 2·5 μg/ml. for NA and less than 0·75 μg/ml. for adrenaline) any catecholamine taken up by this process is rapidly metabolized. Thus the accumulation of unchanged amine is seen only at high perfusion concentrations.
6. The relevance of these results to an understanding of the possible physiological and pharmacological importance of Uptake2 is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avakian O. V., Gillespie J. S. Uptake of noradrenaline by adrenergic nerves, smooth muscle and connective tissue in isolated perfused arteries and its correlation with the vasoconstrictor response. Br J Pharmacol Chemother. 1968 Jan;32(1):168–184. doi: 10.1111/j.1476-5381.1968.tb00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROUT J. R., CREVELING C. R., UDENFRIEND S. Norepinephrine metabolism in rat brain and heart. J Pharmacol Exp Ther. 1961 Jun;132:269–277. [PubMed] [Google Scholar]
- Callingham B. A., Burgen A. S. The uptake of isoprenaline and noradrenaline by the perfused rat heart. Mol Pharmacol. 1966 Jan;2(1):37–42. [PubMed] [Google Scholar]
- Ehinger B., Sporrong B. Neuronal and extraneuronal localization of nonadrenaline in the rat heart after perfusion at high concentration. Experientia. 1968 Mar 15;24(3):265–266. doi: 10.1007/BF02152811. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. J., Axelrod J., Krakoff L. Inhibition of the extraneuronal accumulation and metabolism of norepinephrine by adrenergic blocking agents. J Pharmacol Exp Ther. 1967 Apr;156(1):107–113. [PubMed] [Google Scholar]
- Eisenfeld A. J., Landsberg L., Axelrod J. Effect of drugs on the accumulation and metabolism of extraneuronal norepinephrine in the rat heart. J Pharmacol Exp Ther. 1967 Dec;158(3):378–385. [PubMed] [Google Scholar]
- Farnebo L. O., Malmfors T. Histochemical studies on the uptake of noradrenaline and alpha-methyl-noradrenaline in the perfused rat heart. Eur J Pharmacol. 1969 Mar;5(4):313–320. doi: 10.1016/0014-2999(69)90107-1. [DOI] [PubMed] [Google Scholar]
- Foster R. W. The potentiation of the responses to noradrenaline and isoprenaline of the guinea-pig isolated tracheal chain preparation by desipramine, cocaine, phentolamine, phenoxybenzamine, guanethidine, metanephrine and cooling. Br J Pharmacol Chemother. 1967 Nov;31(3):466–482. doi: 10.1111/j.1476-5381.1967.tb00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVERSEN L. L. THE UPTAKE OF NORADRENALINE BY THE ISOLATED PERFUSED RAT HEART. Br J Pharmacol Chemother. 1963 Dec;21:523–537. doi: 10.1111/j.1476-5381.1963.tb02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Langer S. Z. Effects of phenoxybenzamine on the uptake and metabolism of noradrenaline in the rat heart and vas deferens. Br J Pharmacol. 1969 Nov;37(3):627–637. doi: 10.1111/j.1476-5381.1969.tb08501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S., Nickerson M. Disposition of norepinephrine and epinephrine in vascular tissue, determined by the technique of oil immersion. J Pharmacol Exp Ther. 1969 Feb;165(2):152–165. [PubMed] [Google Scholar]
- Langer S. Z. The effects of phenoxybenzamine on metabolism of 3H-noradrenaline released from the isolated nictitating membrane. Br J Pharmacol. 1968 Sep;34(1):222P–223P. [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z., Trendelenburg U. The effect of a saturable uptake mechanism on the slopes of dose-response curves for sympathomimetic amines and on the shifts of dose-response curves produced by a competitive antagonist. J Pharmacol Exp Ther. 1969 May;167(1):117–142. [PubMed] [Google Scholar]
- MCCAMAN R. E., MCCAMAN M. W., HUNT J. M., SMITH M. S. MICRODETERMINATION OF MONOAMINE OXIDASE AND 5-HYDROXYTRYPTOPHAN DECARBOXYLASE ACTIVITIES IN NERVOUS TISSUES. J Neurochem. 1965 Jan;12:15–23. doi: 10.1111/j.1471-4159.1965.tb10246.x. [DOI] [PubMed] [Google Scholar]
- Maxwell R. A., Wastila W. B., Eckhardt S. B. Some factors determining the response of rabbit aortic strips to dl-norepinephrine-7-H3 hydrochloride and the influence of cocaine, guanethidine and methylphenidate on these factors. J Pharmacol Exp Ther. 1966 Feb;151(2):253–261. [PubMed] [Google Scholar]
- Maître L. Monoamine oxidase inhibiting properties of SU-11,739 in the rat. Comparison with pargyline, tranylcypromine and iproniazid. J Pharmacol Exp Ther. 1967 Jul;157(1):81–88. [PubMed] [Google Scholar]
- McCaman R. E. Microdetermination of catechol-O-methyl transferase in brain. Life Sci. 1965 Dec;4(24):2353–2359. doi: 10.1016/0024-3205(65)90290-0. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U., MUSKUS A., FLEMING W. W., de la GOMEZ ALONSO SIERRA B. Effect of cocaine, denervation and decentralization on the response of the nictitating membrane to various sympathomimetic amines. J Pharmacol Exp Ther. 1962 Nov;138:181–193. [PubMed] [Google Scholar]
- Vane J. R. The release and fate of vaso-active hormones in the circulation. Br J Pharmacol. 1969 Feb;35(2):209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


