Abstract
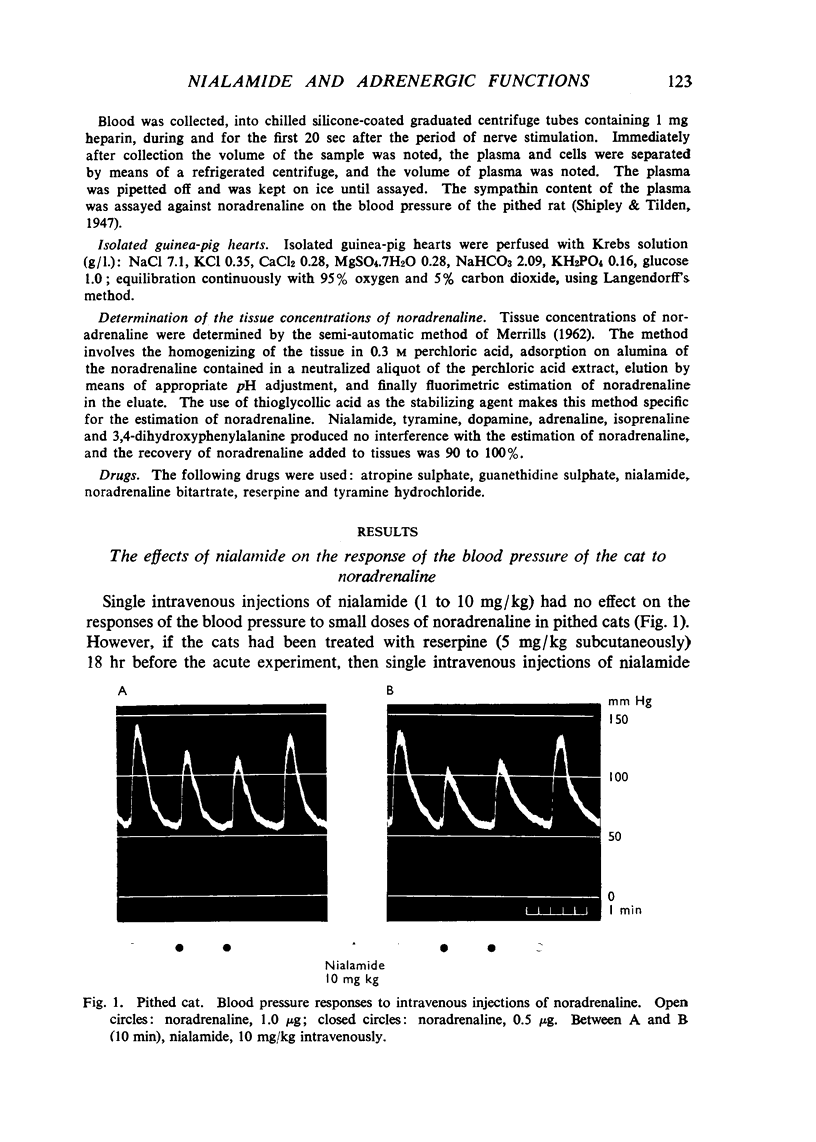
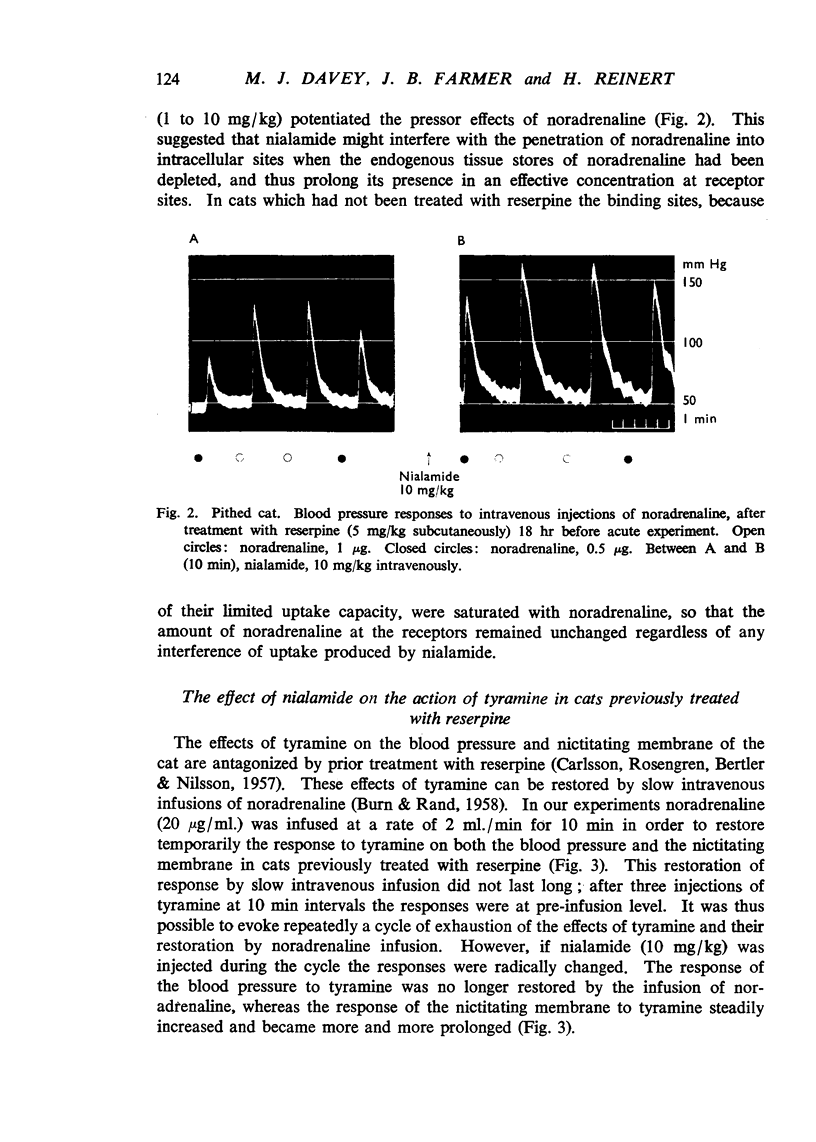
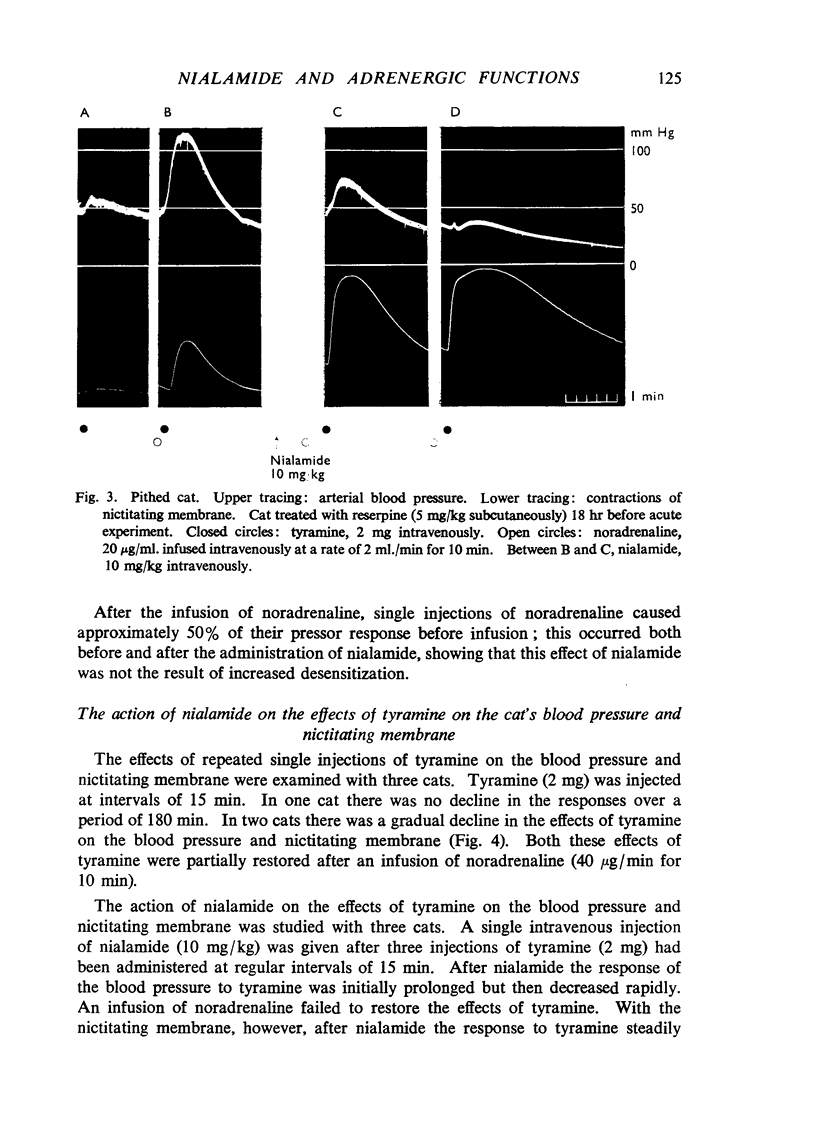
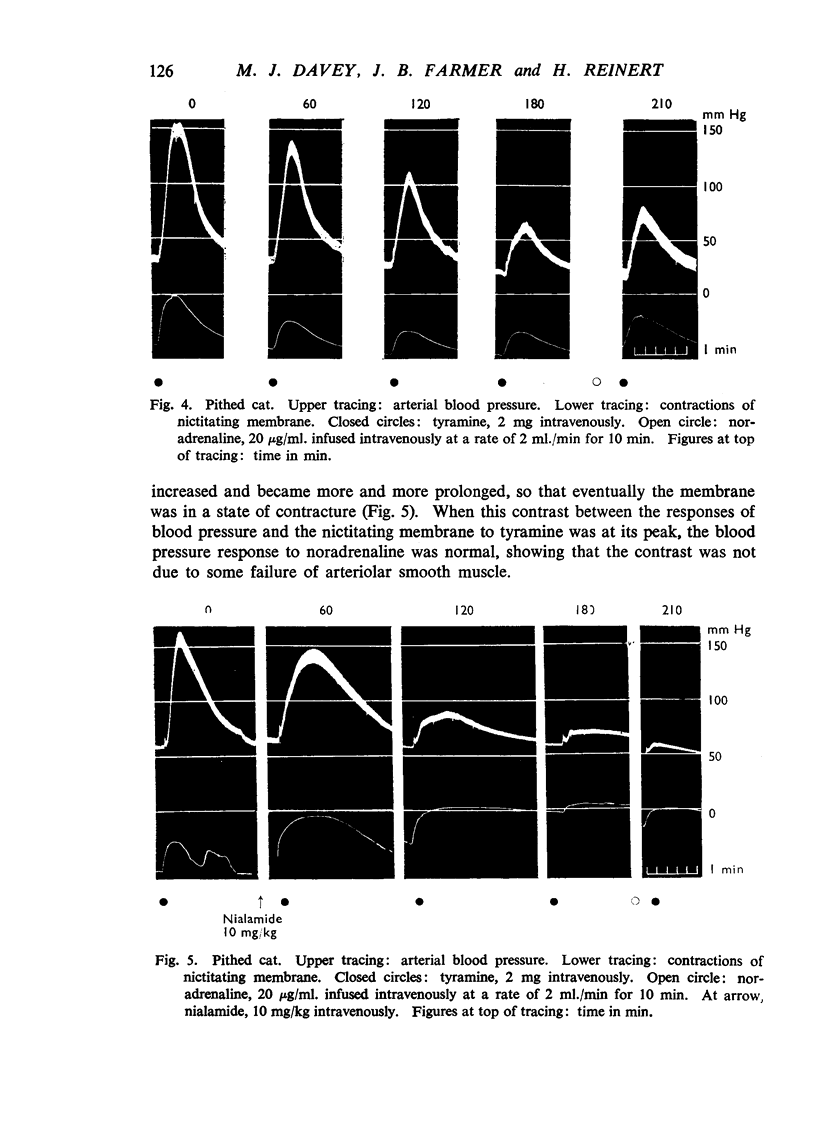
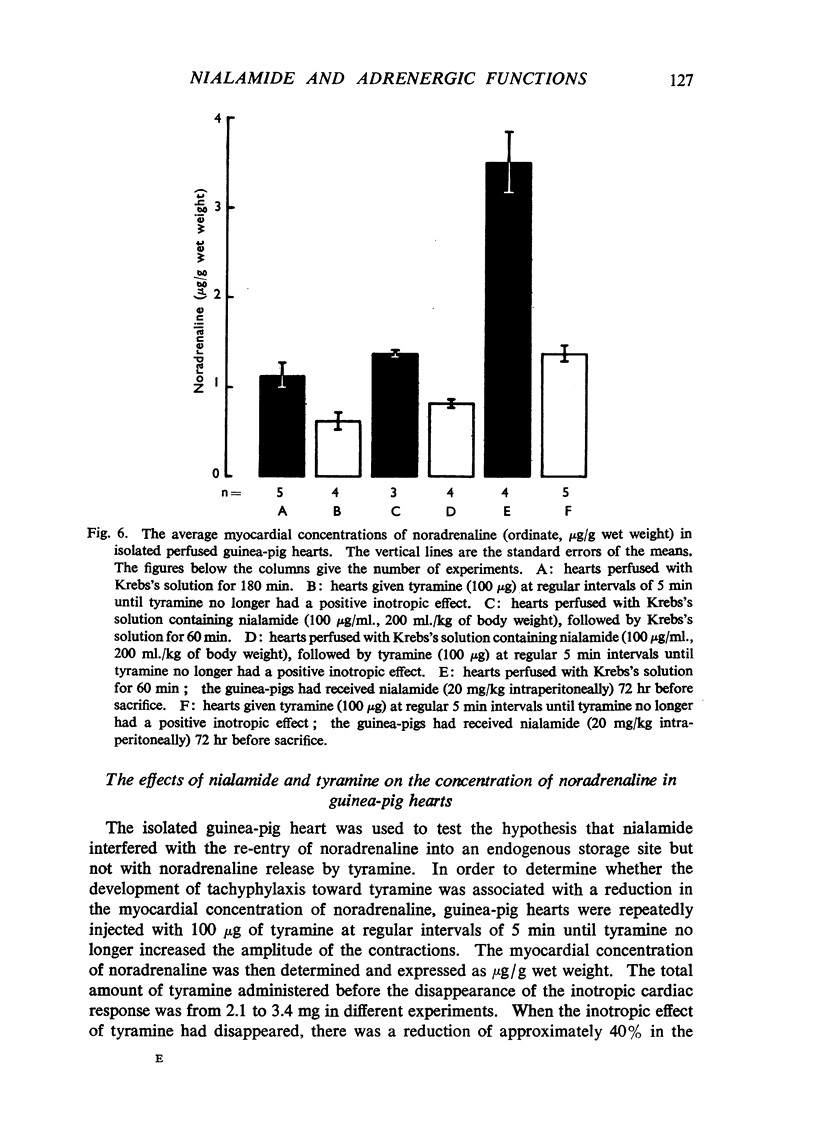
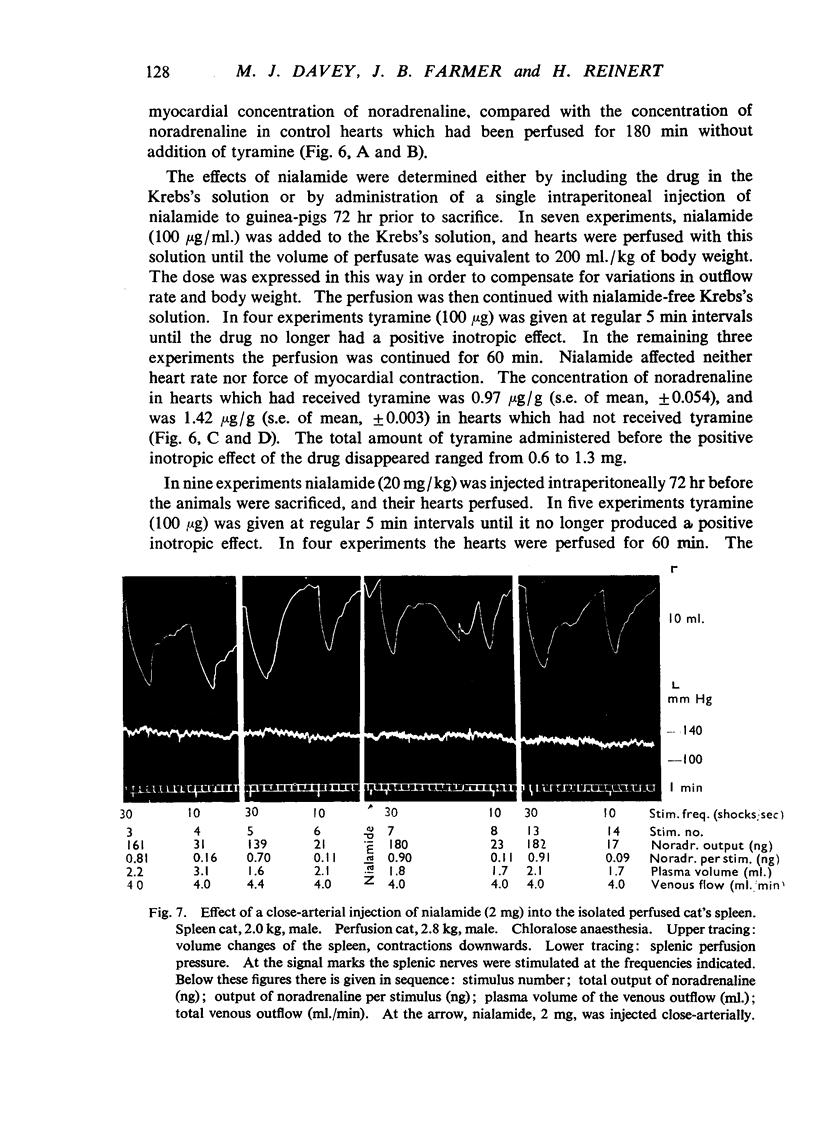
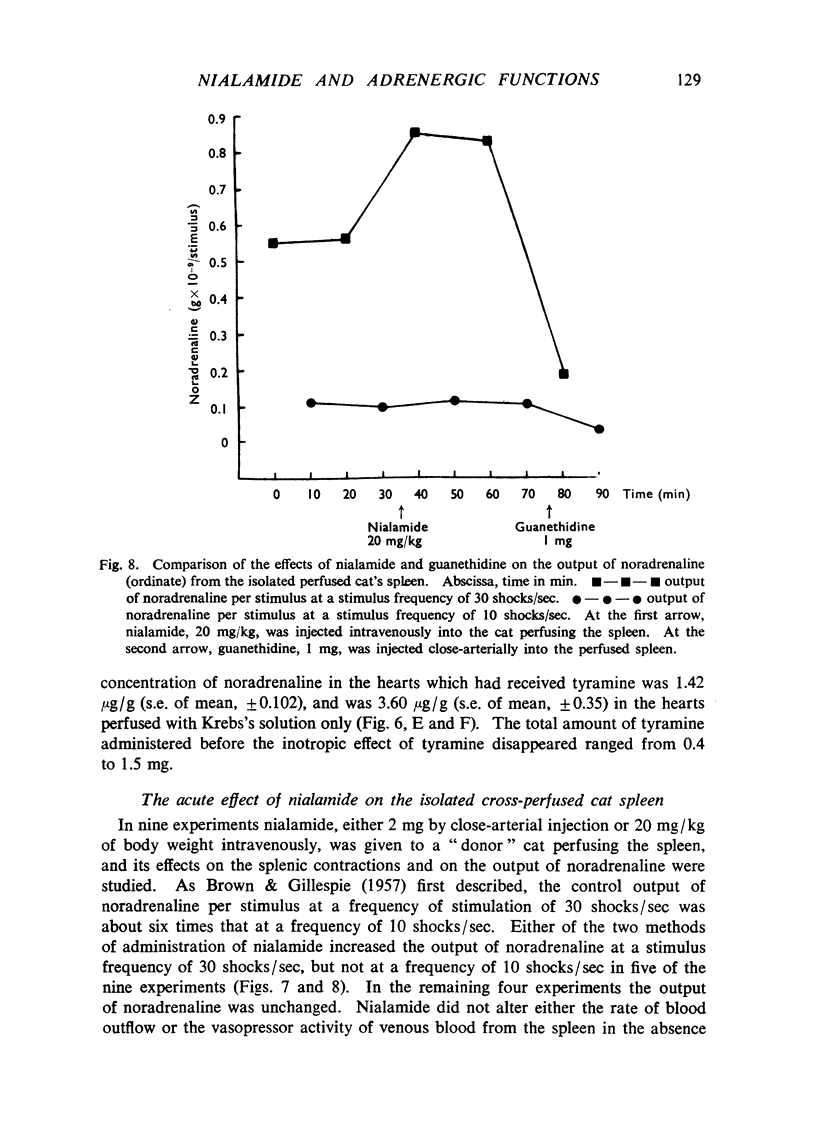
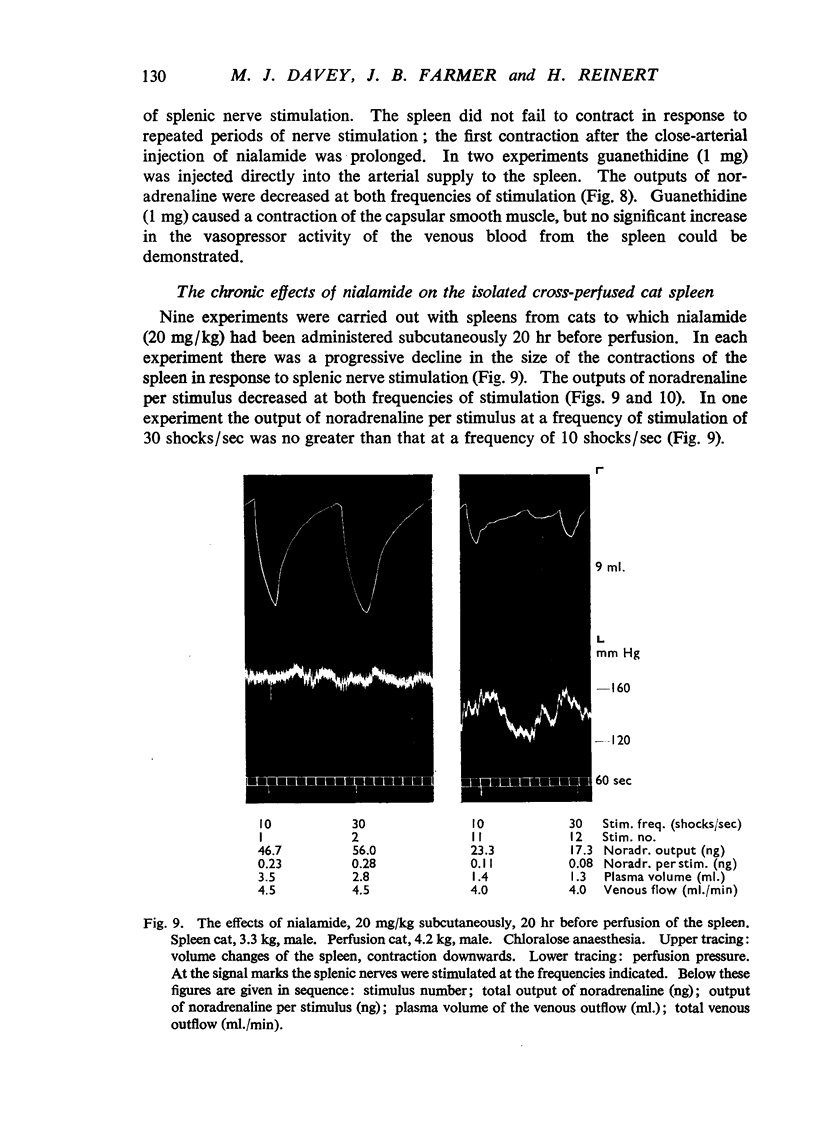
Nialamide potentiated the pressor effects of noradrenaline in the pithed cat. In cats treated with reserpine and then pithed, it prevented the restoration of the pressor effects of tyramine by slow intravenous infusions of noradrenaline. Nialamide produced a gradual decline in the pressor effects of repeated injections of tyramine whilst the effects of tyramine on the nictitating membrane were potentiated. The development of tachyphylaxis to tyramine in the isolated guinea-pig heart was associated with a 40% reduction in the myocardial concentration of noradrenaline. The onset of tachyphylaxis to tyramine was more rapid when nialamide was either included in the perfusion fluid or administered in vivo. Prior treatment with nialamide increased threefold the myocardial concentration of noradrenaline; however, the development of tachyphylaxis to tyramine was associated with a proportionate fall in the myocardial concentration of noradrenaline. In five out of nine experiments the acute administration of nialamide increased the output of noradrenaline per stimulus from the isolated cross-perfused spleen of the cat when the stimulus frequency was 30 shocks/sec, but not when the frequency was 10 shocks/sec. When nialamide (20 mg/kg) was given subcutaneously to cats 20 hr before their spleens were isolated and perfused, there was a rapid fall-off in the contractions of the spleen in response to periods of nerve stimulation. The outputs of noradrenaline per stimulus were decreased at both frequencies of stimulation. Nialamide decreased the concentrations of noradrenaline in the myocardium and spleen of the cat. The hypothesis is proposed that nialamide diminishes the availability of transmitter, as a consequence of a decreased re-entry of noradrenaline into a storage site present in nerve endings, and that such a decrease in the availability of noradrenaline in hyperactive nerve pathways may account for the antihypertensive effects of monoamine oxidase inhibitors in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. Sympathetic postganglionic cholinergic fibres. Br J Pharmacol Chemother. 1960 Mar;15:56–66. doi: 10.1111/j.1476-5381.1960.tb01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The action of sympathomimetic amines in animals treated with reserpine. J Physiol. 1958 Dec 4;144(2):314–336. doi: 10.1113/jphysiol.1958.sp006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALY M. D., SCOTT M. J. The effects of acetylcholine on the volume and vascular resistance of the dog's spleen. J Physiol. 1961 Apr;156:246–259. doi: 10.1113/jphysiol.1961.sp006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVEY M. J., FARMER J. B., REINERT H. Hypotension and monoamineoxydase inhibitors. Chemotherapia (Basel) 1962;4:314–328. doi: 10.1159/000219926. [DOI] [PubMed] [Google Scholar]
- GERTNER S. B. The effects of monoamine oxidase inhibitors on ganglionic transmission. J Pharmacol Exp Ther. 1961 Feb;131:223–230. [PubMed] [Google Scholar]
- GILLESPIE J. S., MACKENNA B. R. The inhibitory action of the sympathetic nerves on the smooth muscle of the rabbit gut, its reversal by reserpine and restoration by catechol amines and by DOPA. J Physiol. 1961 Apr;156:17–34. doi: 10.1113/jphysiol.1961.sp006655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG N. D., SHIDEMAN F. E. Species differences in the cardiac effects of a monoamine oxidase inhibitor. J Pharmacol Exp Ther. 1962 May;136:142–151. [PubMed] [Google Scholar]
- HERTTING G., AXELROD J. Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature. 1961 Oct 14;192:172–173. doi: 10.1038/192172a0. [DOI] [PubMed] [Google Scholar]
- MARLEY E., PATON W. D. The output of sympathetic amines from the cat's adrenal gland in response to splanchnic nerve activity. J Physiol. 1961 Jan;155:1–27. doi: 10.1113/jphysiol.1961.sp006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRILLS R. J. An autoanalytical method for the estimation of adrenaline and noradrenaline. Nature. 1962 Mar 10;193:988–988. doi: 10.1038/193988a0. [DOI] [PubMed] [Google Scholar]


