Abstract
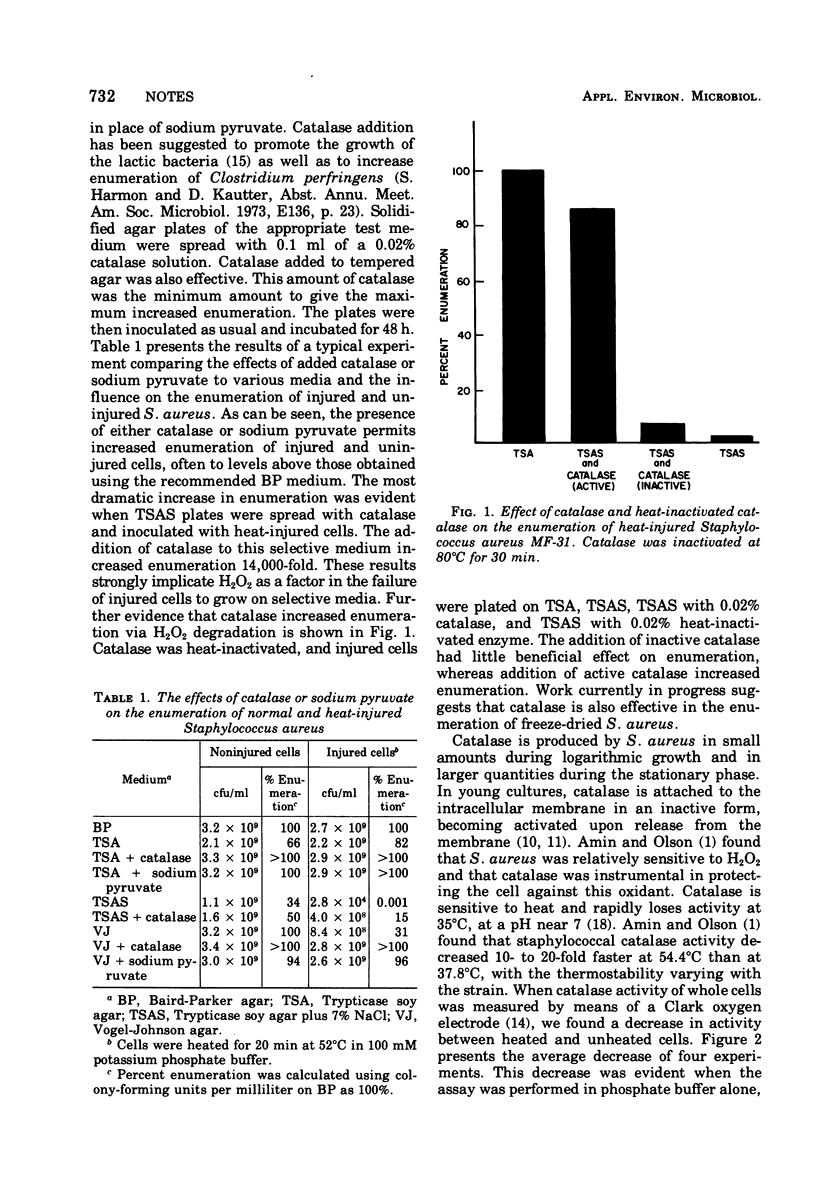
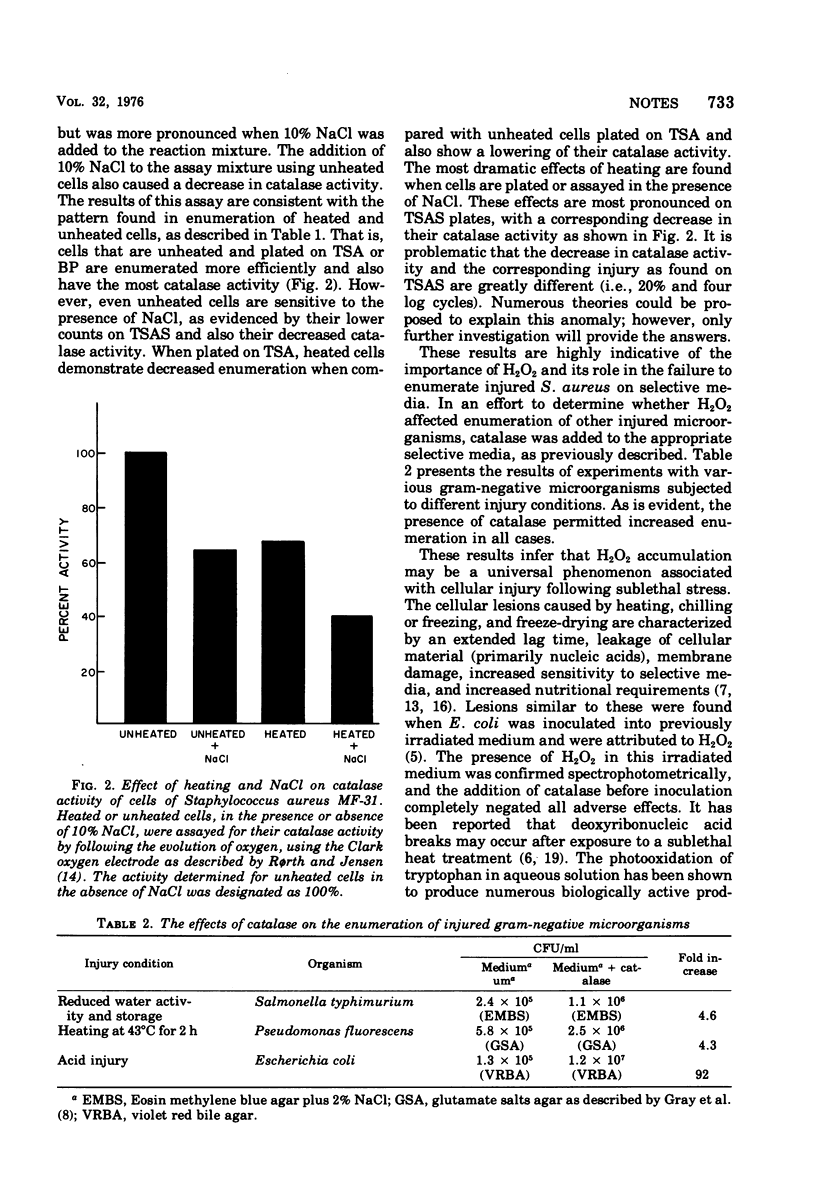
The addition of catalase to the surface of selective medium plates permitted increased enumeration of physically or chemically injured (stressed) microorganisms. Catalase acted by preventing the accumulation of hydrogen peroxide in, or around, injured cells. Heat-injured Staphylococcus aureus cells had decreased catalase activity, and heat-inactivated catalase had no effect on enumeration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin V. M., Olson N. F. Influence of catalase activity on resistance of coagulase-positive staphylococci to hydrogen peroxide. Appl Microbiol. 1968 Feb;16(2):267–270. doi: 10.1128/am.16.2.267-270.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer E. F., Gilden M. M., Wienke C. L., Mellitz M. B. Comparative efficiency of two enrichment and four plating media for isolation of Staphylococcus aureus. J Assoc Off Anal Chem. 1971 May;54(3):736–738. [PubMed] [Google Scholar]
- Bairdparker A. C., Davenport E. The effect of recovery medium on the isolation of Staphylococcus aureus after heat treatment and after the storage of frozen or dried cells. J Appl Bacteriol. 1965 Dec;28(3):390–402. doi: 10.1111/j.1365-2672.1965.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Frey H. E., Pollard E. C. The action of gamma-ray-irradiated medium on bacteria: relation to the electron transport system. Radiat Res. 1968 Oct;36(1):59–67. [PubMed] [Google Scholar]
- Gomez R. F., Sinskey A. J. Deoxyribonucleic acid breaks in heated Salmonella typhimurium LT-2 after exposure to nutritionally complex media. J Bacteriol. 1973 Aug;115(2):522–528. doi: 10.1128/jb.115.2.522-528.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R., Takano M., Sinskey A. J. Proceedings: Characteristics of freeze-dried cells. Cryobiology. 1973 Nov;10(5):368–374. doi: 10.1016/0011-2240(73)90060-6. [DOI] [PubMed] [Google Scholar]
- Gray R. J., Witter L. D., Ordal Z. J. Characterization of mild thermal stress in Pseudomonas fluorescens and its repair. Appl Microbiol. 1973 Jul;26(1):78–85. doi: 10.1128/am.26.1.78-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács E., Lantos J., Mazarean H. H. The effect of phage infection on the catalase induction of the Staphylococcus aureus culture. Experientia. 1966 Dec 15;22(12):802–803. doi: 10.1007/BF01897425. [DOI] [PubMed] [Google Scholar]
- McCormick J. P., Fischer J. R., Pachlatko J. P., Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science. 1976 Feb 6;191(4226):468–469. doi: 10.1126/science.1108203. [DOI] [PubMed] [Google Scholar]
- Ray B., Speck M. L. Freeze-injury in bacteria. CRC Crit Rev Clin Lab Sci. 1973 Aug;4(2):161–213. doi: 10.3109/10408367309151556. [DOI] [PubMed] [Google Scholar]
- Rorth M., Jensen P. K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967 May 16;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- Woodcock E., Grigg G. W. Repair of thermally induced DNA breakage in Escherichia coli. Nat New Biol. 1972 May 17;237(72):76–79. doi: 10.1038/newbio237076a0. [DOI] [PubMed] [Google Scholar]


