Abstract
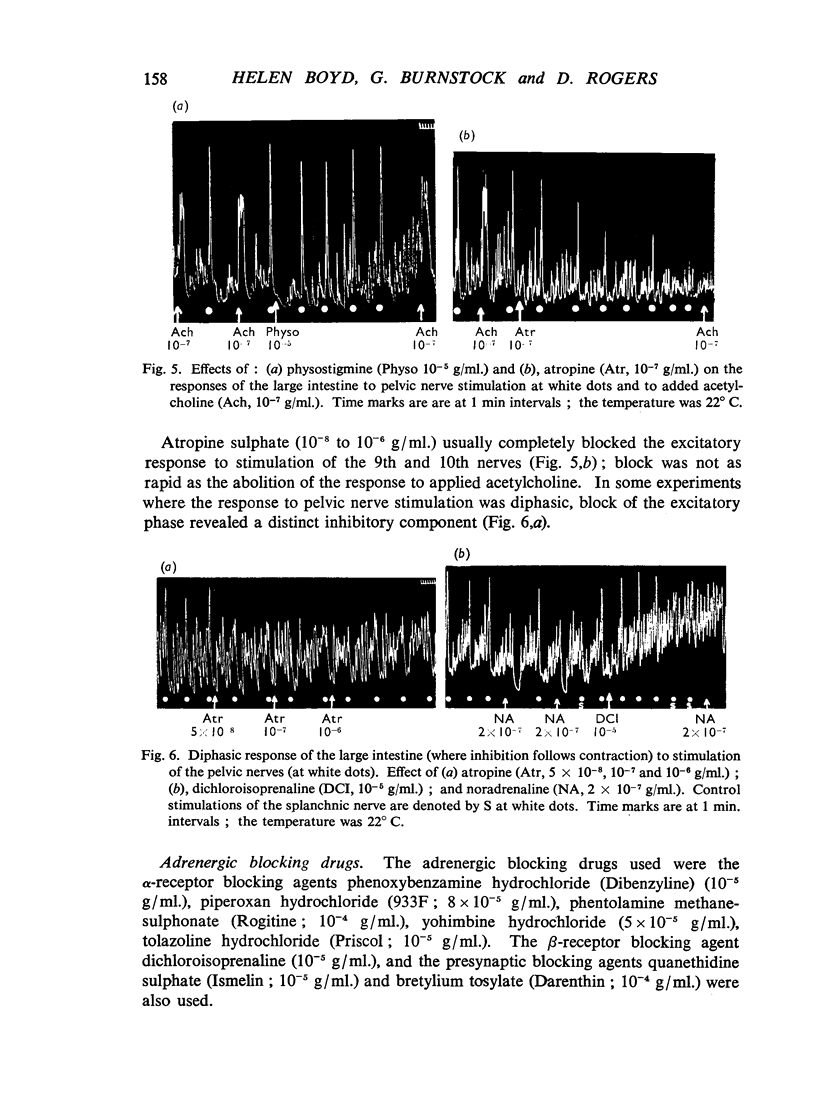
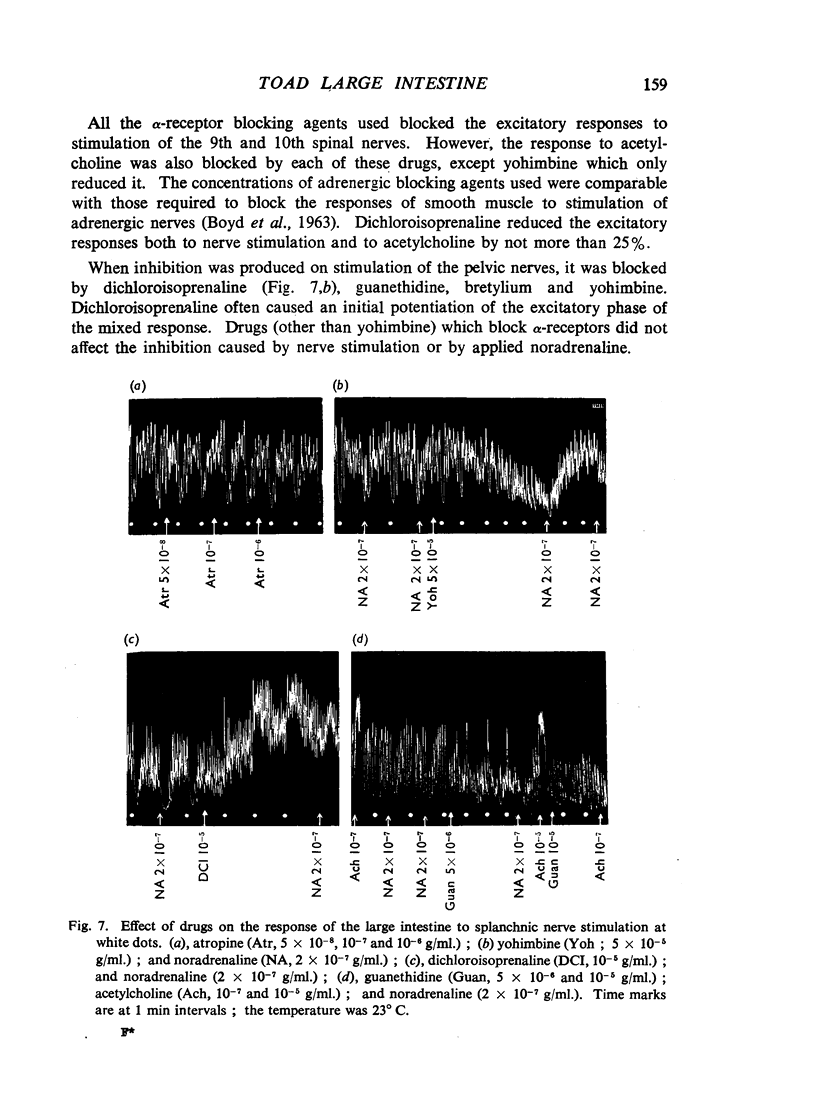
The morphology, physiology and pharmacology of the innervation of the toad (Bufo marinus) large intestine have been studied. The large intestine can be divided into the regions colon, rectum and cloaca, on morphological grounds, but acts as a unit in response to nerve stimulation. Of the right and left nerves, each appears to supply the entire large intestine. Autonomic innervation of the large intestine of Bufo marinus is as follows: (1) The 9th and 10th spinal nerves (pelvic) contain predominantly excitatory preganglionic cholinergic fibres, but some inhibitory adrenergic fibres are also present in most preparations. (2) The splanchnic nerves contain inhibitory postganglionic adrenergic fibres from the 3rd to 5th sympathetic ganglia, and a small number of excitatory cholinergic fibres. The pathway of adrenergic inhibitory fibres to the large intestine alongside the posterior mesenteric artery as seen in mammals is rarely present in the toad. Several nonspecific actions of autonomic drugs on the large intestine are discussed. The functional organization of the autonomic innervation of the toad large intestine is similar to that in mammals, that is the large intestine is controlled by antagonistic cholinergic and adrenergic nerves. However, the separation of these two types of nerve fibres into anatomically distinct nerves does not appear to be as complete as in mammals. It is suggested that inhibitory autonomic control of the alimentary canal in vertebrates first appears in the hind-gut region.
Full text
PDF

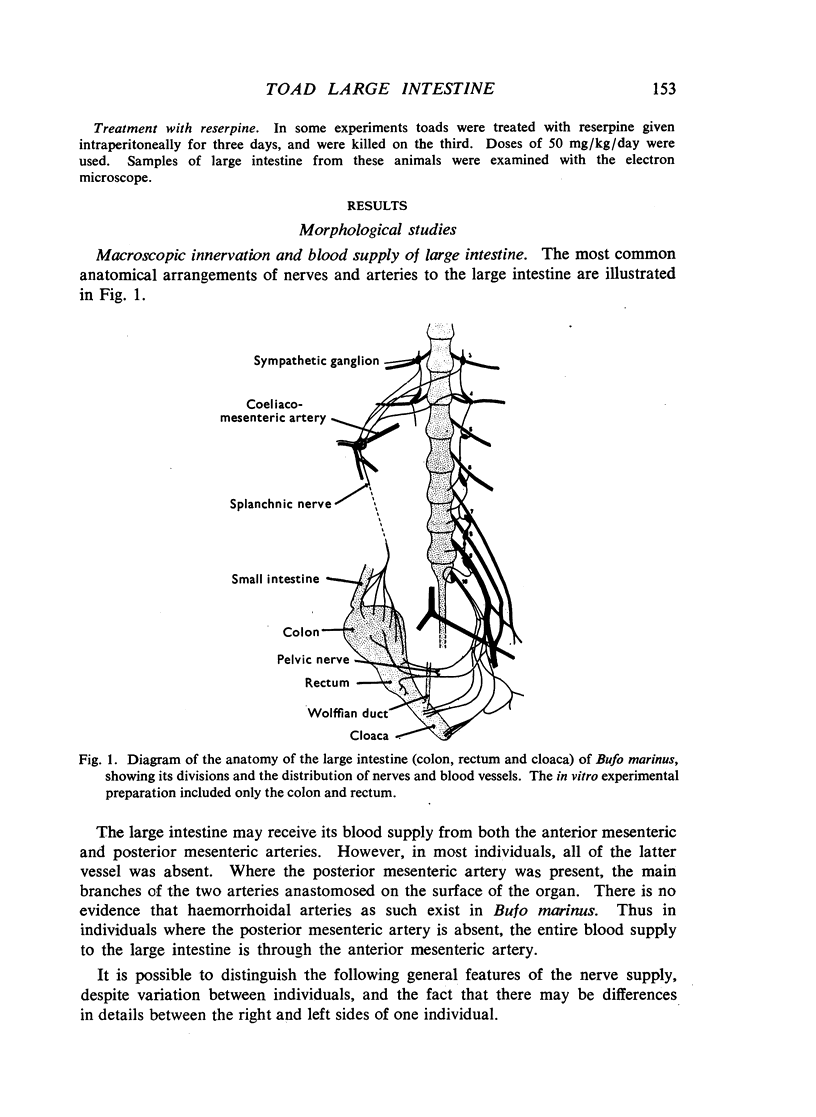

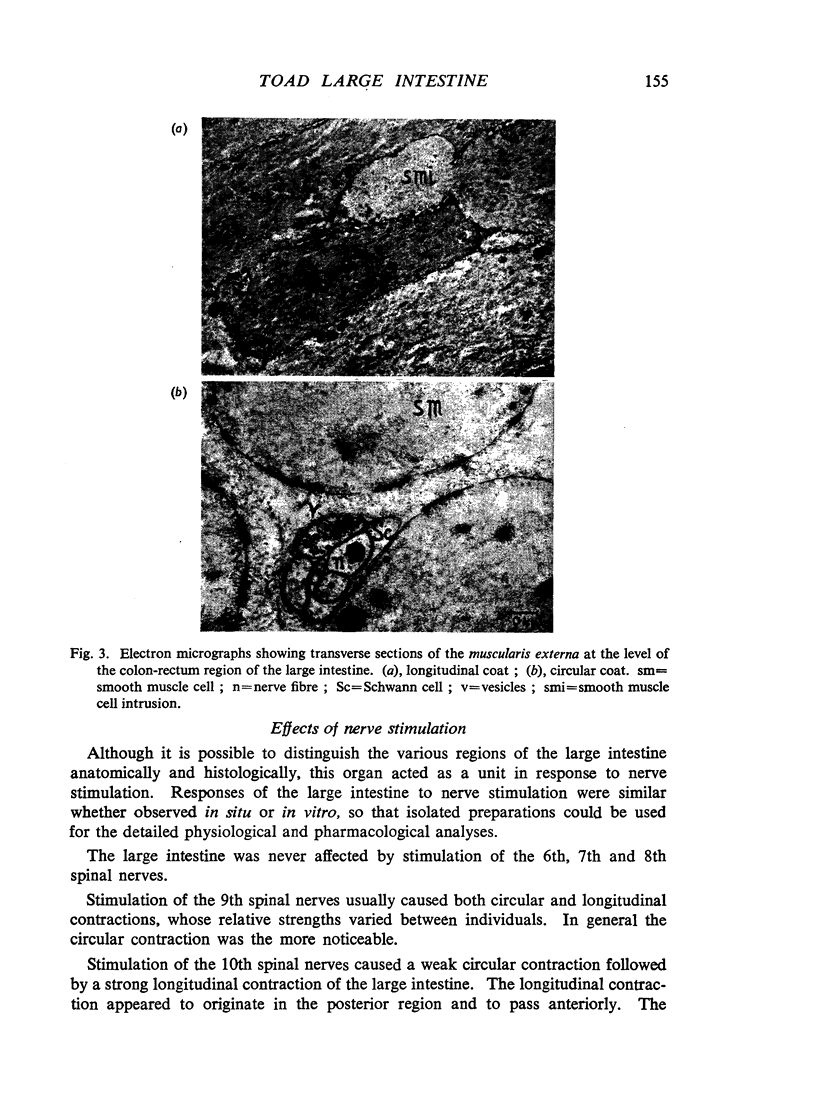
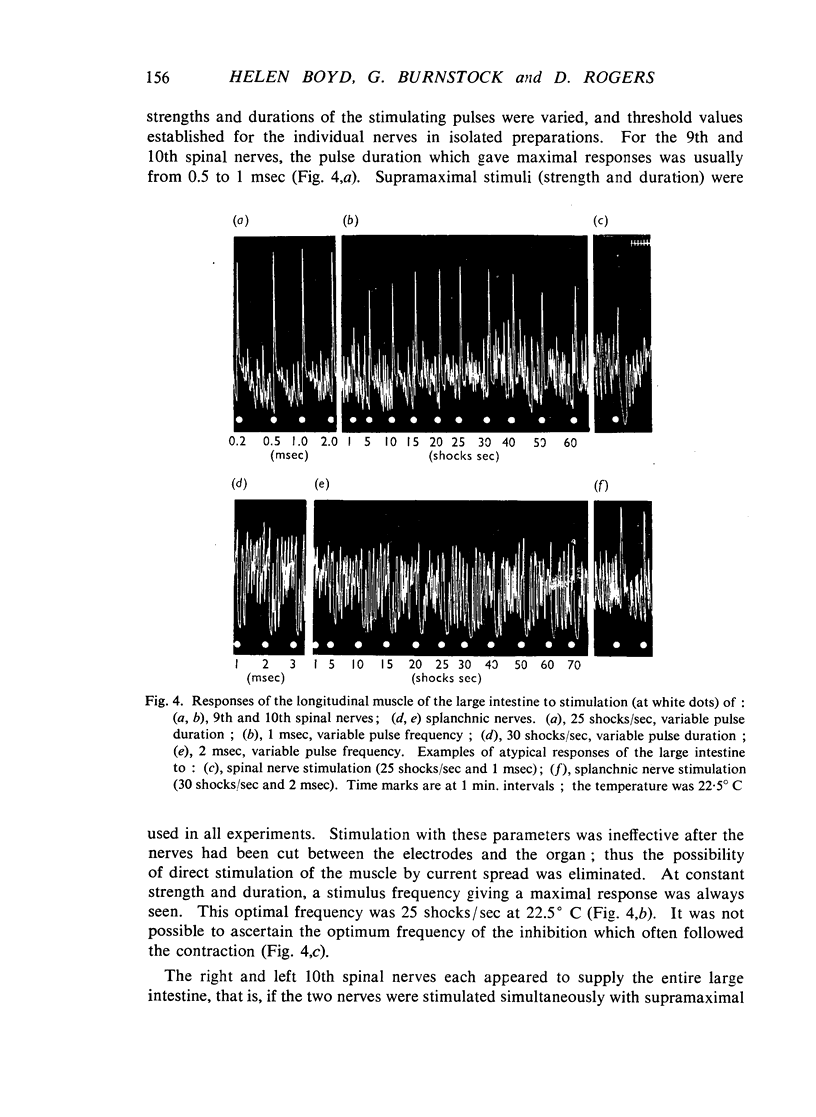





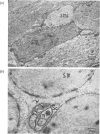
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD H., BURNSTOCK G., CAMPBELL G., JOWETT A., O'SHEA J., WOOD M. The cholinergic blocking action of adrenergic blocking agents in the pharmacological analysis of autonomic innervation. Br J Pharmacol Chemother. 1963 Jun;20:418–435. doi: 10.1111/j.1476-5381.1963.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The relation of circulating noradrenaline to the effect of sympathetic stimulation. J Physiol. 1960 Feb;150:295–305. doi: 10.1113/jphysiol.1960.sp006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G. COMPARATIVE PHYSIOLOGY OF THE VERTEBRATE AUTONOMIC NERVOUS SYSTEM. II. INNERVATION OF THE URINARY BLADDER OF THE RINGTAIL POSSUM (PSEUDOCHEIRUS PEREGRINUS). J Exp Biol. 1963 Sep;40:421–436. doi: 10.1242/jeb.40.3.421. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., O'SHEA J., WOOD M. COMPARATIVE PHYSIOLOGY OF THE VERTEBRATE AUTONOMIC NERVOUS SYSTEM. I. INNERVATION OF THE URINARY BLADDER OF THE TOAD (BUFO MARINUS). J Exp Biol. 1963 Sep;40:403–419. doi: 10.1242/jeb.40.3.403a. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. The effect of drugs on spontaneous motility and on response to stimulation of the extrinsic nerves of the gut of a teleostean fish. Br J Pharmacol Chemother. 1958 Sep;13(3):216–226. doi: 10.1111/j.1476-5381.1958.tb00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRY R. C., GILLESPIE J. S. The responses of the musculature of the colon of the rabbit to stimulation, in vitro, of the parasympathetic and of the sympathetic outflows. J Physiol. 1955 Jun 28;128(3):557–576. doi: 10.1113/jphysiol.1955.sp005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S., MACKENNA B. R. The inhibitory action of the sympathetic nerves on the smooth muscle of the rabbit gut, its reversal by reserpine and restoration by catechol amines and by DOPA. J Physiol. 1961 Apr;156:17–34. doi: 10.1113/jphysiol.1961.sp006655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton-Smith R. J. On Efferent Fibres in the Posterior Roots of the Frog. J Physiol. 1897 Mar 17;21(2-3):101–111. doi: 10.1113/jphysiol.1897.sp000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Orbeli L. A. Observations on the sympathetic and sacral autonomic system of the frog. J Physiol. 1910 Dec 31;41(5):450–482. doi: 10.1113/jphysiol.1910.sp001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON D. F., WIEN R. The actions of heterocyclic bisquaternary compounds, especially of a pyrrolidinium series. Br J Pharmacol Chemother. 1955 Mar;10(1):124–132. doi: 10.1111/j.1476-5381.1955.tb00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCHOLL E., VOGT M. The action of reserpine on the peripheral sympathetic system. J Physiol. 1958 Apr 3;141(1):132–155. doi: 10.1113/jphysiol.1958.sp005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]