Abstract
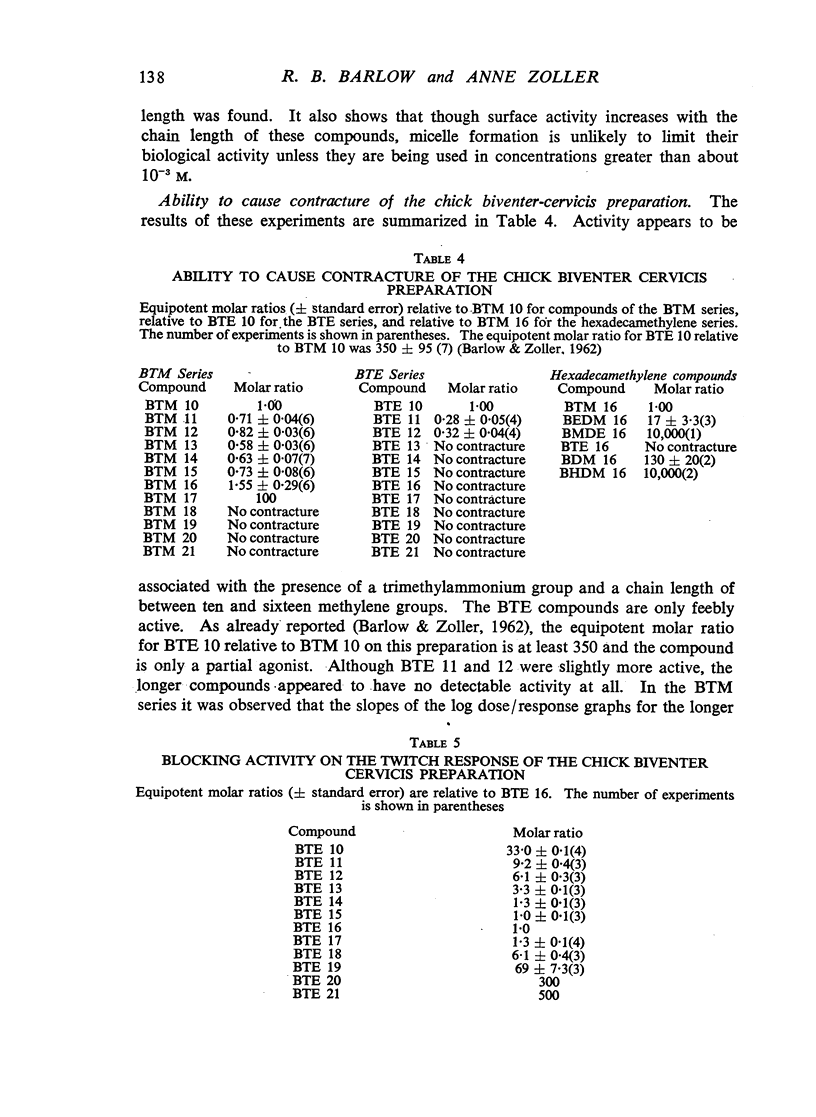
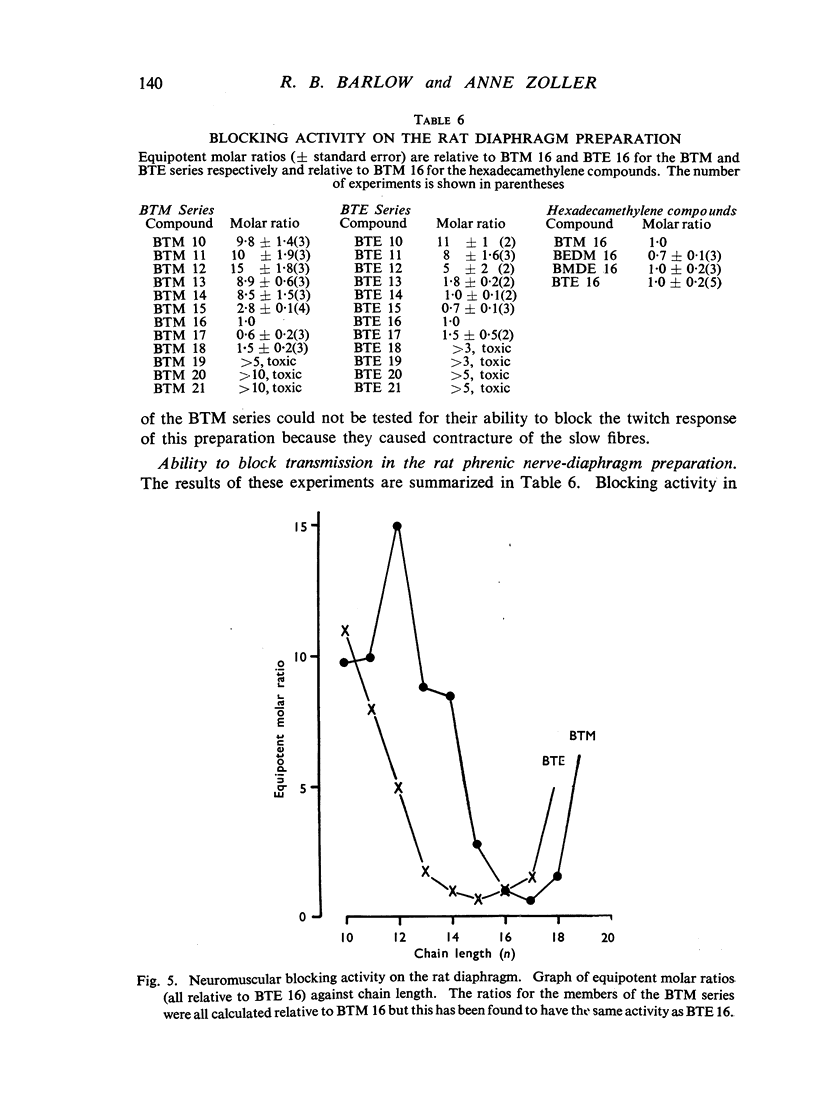
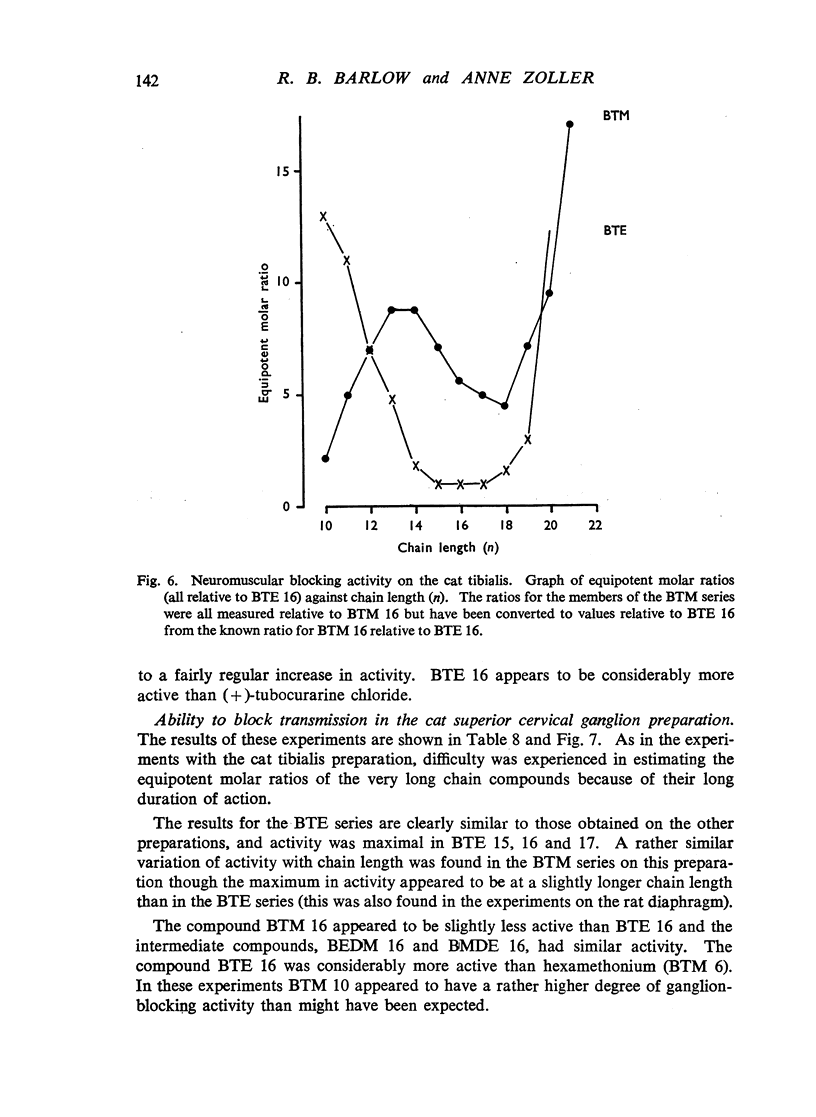
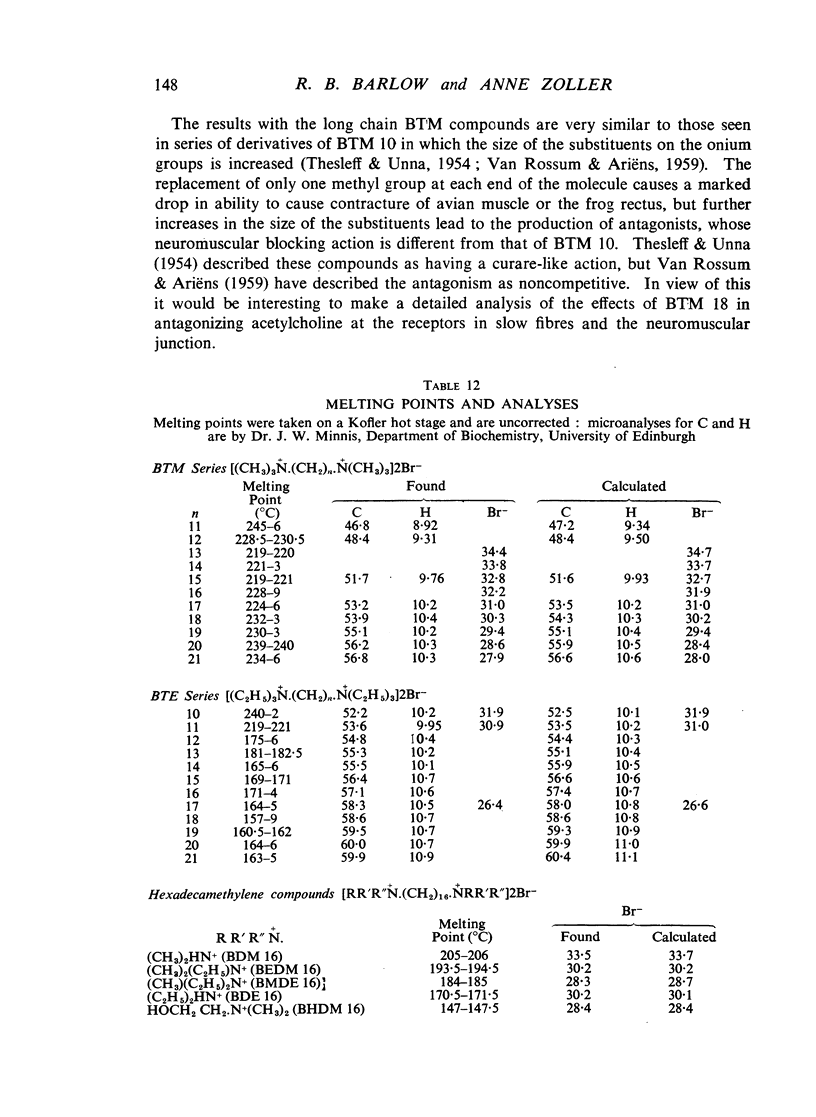
A survey has been made of the effects on junctional transmission of the complete series of polymethylene bis-trimethylammonium (BTM) and bis-triethylammonium (BTE) salts from the decamethylene compounds (BTM 10 and BTE 10) to those with twenty-one methylene groups in the chain. These were tested for their ability to cause contracture of the isolated chick biventer cervicis preparation, and for their ability to block the twitch responses of this preparation, those of the rat isolated diaphragm preparation, and those of the cat tibialis anterior preparation. They were also tested for their ability to block transmission in the cat superior cervical ganglion, to block the actions of acetylcholine on the guinea-pig isolated ileum, and for ability to inhibit the hydrolysis of acetylcholine by acetylcholinesterase. Their electrical conductivity has been measured in aqueous solution. Ability to cause contracture of the chick biventer cervicis is confined to the compounds BTM 10 to 15; BTE 10, 11 and 12 have some weak activity but the other BTE compounds, and the BTM compounds with more than fifteen methylene groups, have virtually no activity. In the BTE series both neuromuscular blocking and ganglion-blocking activities increase with chain length up to a maximum in the region of BTE 15 to 17 and then decline. In the BTM series ganglion-blocking activity increases with chain length in much the same way as in the BTE series, though the maximum activity is at a slightly longer chain length. At the neuromuscular junction an increase in chain length beyond BTM 10 leads to a decline in activity but this returns to some extent at longer chain lengths, reaching a second maximum at BTM 18, above which it declines further. At the ganglion BTE 16 is only slightly more active than BTM 16 and about five-times as active as hexamethonium; at the neuromuscular junction in the cat BTE 16 is about five-times as active as BTM 16 and about eight-times as active as (+)-tubocurarine. The affinity of the BTE compounds for the postganglionic acetylcholine receptors of the guinea-pig ileum reaches a maximum at BTE 14 but does not decline significantly with further increase in chain length. Anticholinesterase activity, likewise, does not alter significantly between BTM 12 and BTM 21 and the activity of the compounds in the BTE series appears to be similar. This property could conceivably be modifying the actions of some of the intermediate compounds but is not likely to be affecting those of the more active ones. The conductivity experiments indicate that micelle formation could be limiting the actions of the compounds with 20 or 21 methylene groups, but is not likely to be affecting those of the other compounds. The results suggest that there is a regular increase with chain length of the affinity of these compounds for the receptors in the ganglia and at the neuromuscular junction but that efficacy in causing contracture is limited to compounds with three methyl groups in the cationic head and a chain of about ten methylene groups. The connexion between this ability to depolarize and the ability to block transmission by desensitization is discussed.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIENS E. J., SIMONIS A. M., DE GROOT W. M. Affinity and intrinsic-activity in the theory of competitive- and non-competitive inhibition and an analysis of some forms of dualism in action. Arch Int Pharmacodyn Ther. 1955 Jan 1;100(3-4):298–322. [PubMed] [Google Scholar]
- BARLOW R. B., HIMMS J. M. The anticholinesterase activity of polymethylene bis-quinolinium salts. Br J Pharmacol Chemother. 1955 Jun;10(2):173–174. doi: 10.1111/j.1476-5381.1955.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW R. B., ROBERTS T. D. M., REID D. A. The neuromuscular blocking activity of ethyl analogues of decamethonium. J Pharm Pharmacol. 1953 Jan;5(1):35–38. doi: 10.1111/j.2042-7158.1953.tb13952.x. [DOI] [PubMed] [Google Scholar]
- BARLOW R. B., ZOLLER A. Activity of analogues of decamethonium on the chick biventer cervicis preparation. Br J Pharmacol Chemother. 1962 Dec;19:485–491. doi: 10.1111/j.1476-5381.1962.tb01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMANN F., SEGAL R. The relationship of quaternary ammonium salts to the anionic sites of true and pseudo cholinesterase. Biochem J. 1954 Dec;58(4):692–698. doi: 10.1042/bj0580692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTILLO J. C., PHILLIPS A. P., DE BEER E. J. The curariform action of decamethylene-1,10-bis-trimethylammonium bromide. J Pharmacol Exp Ther. 1949 Oct;97(2):150–156. [PubMed] [Google Scholar]
- GILL E. W., ING H. R. The problem of hexamethonium. Farmaco Sci. 1958;13(3):244–256. [PubMed] [Google Scholar]
- GINSBORG B. L., WARRINER J. The isolated chick biventer cervicis nerve-muscle preparation. Br J Pharmacol Chemother. 1960 Sep;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINZEL K. H., KLUPP H., WERNER G. Die Wirkung einiger aliphatischer alpha-omega-Bis-quarternärer Ammonium-Verbindungen auf die Skelettmuskulatur. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1951;213(5):453–466. [PubMed] [Google Scholar]
- JEWELL P. A., ZAIMIS E. J. A differentiation between red and white muscle in the cat based on responses to neuromuscular blocking agents. J Physiol. 1954 Jun 28;124(3):417–428. doi: 10.1113/jphysiol.1954.sp005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M., PERRY W. L. M. The relationship between depolarization and block in the cat's superior cervical ganglion. J Physiol. 1953 Jan;119(1):43–57. doi: 10.1113/jphysiol.1953.sp004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S., UNNA K. R. Differences in mode of neuromuscular blockade in a series of symmetric bis-quaternary ammonium salts. J Pharmacol Exp Ther. 1954 May;111(1):99–113. [PubMed] [Google Scholar]
- WIEN R., MASON D. F. J., EDGE N. D., LANGSTON G. T. The ganglion blocking properties of homologous compounds in the methonium series. Br J Pharmacol Chemother. 1952 Dec;7(4):534–541. doi: 10.1111/j.1476-5381.1952.tb00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEN R., MASON D. F. J. Some actions of hexamethonium and certain homologues. Br J Pharmacol Chemother. 1951 Dec;6(4):611–629. doi: 10.1111/j.1476-5381.1951.tb00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


