Abstract
The Robbins-Monro process is a sequential procedure which can be used in toxicity assays. The principal advantages are maximal economy of drug and animals and immediate availability of an estimate of the LD50 at any stage in the assay. The disadvantages are the need to wait for the outcome in each group of animals before testing the next group and the lack of an accurate method for determining confidence limits. Some practical details of the application of the method are given.
Full text
PDF


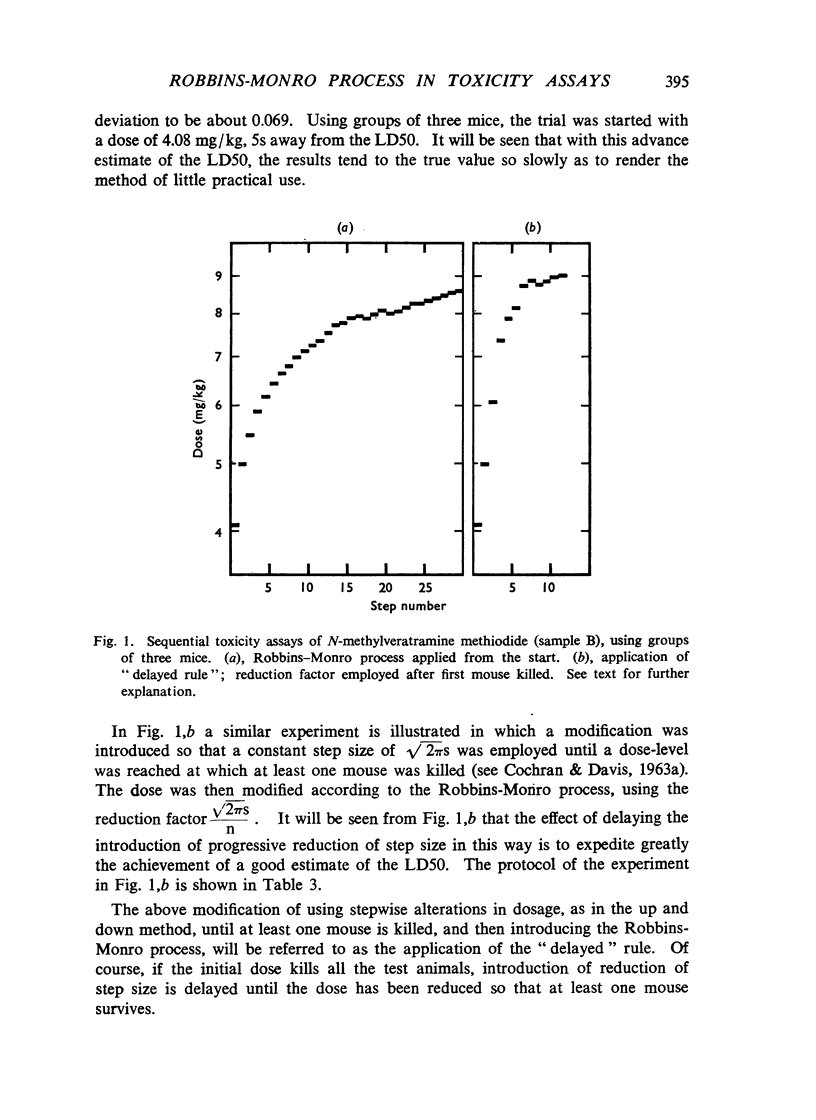
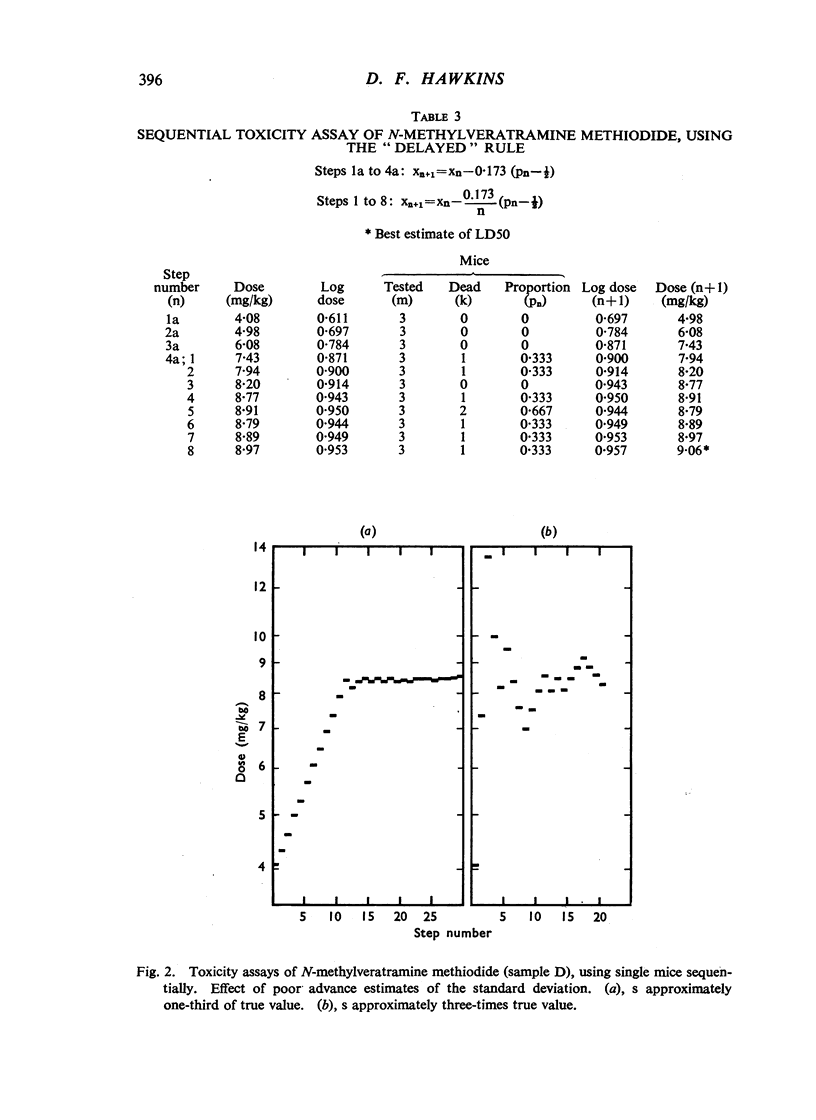
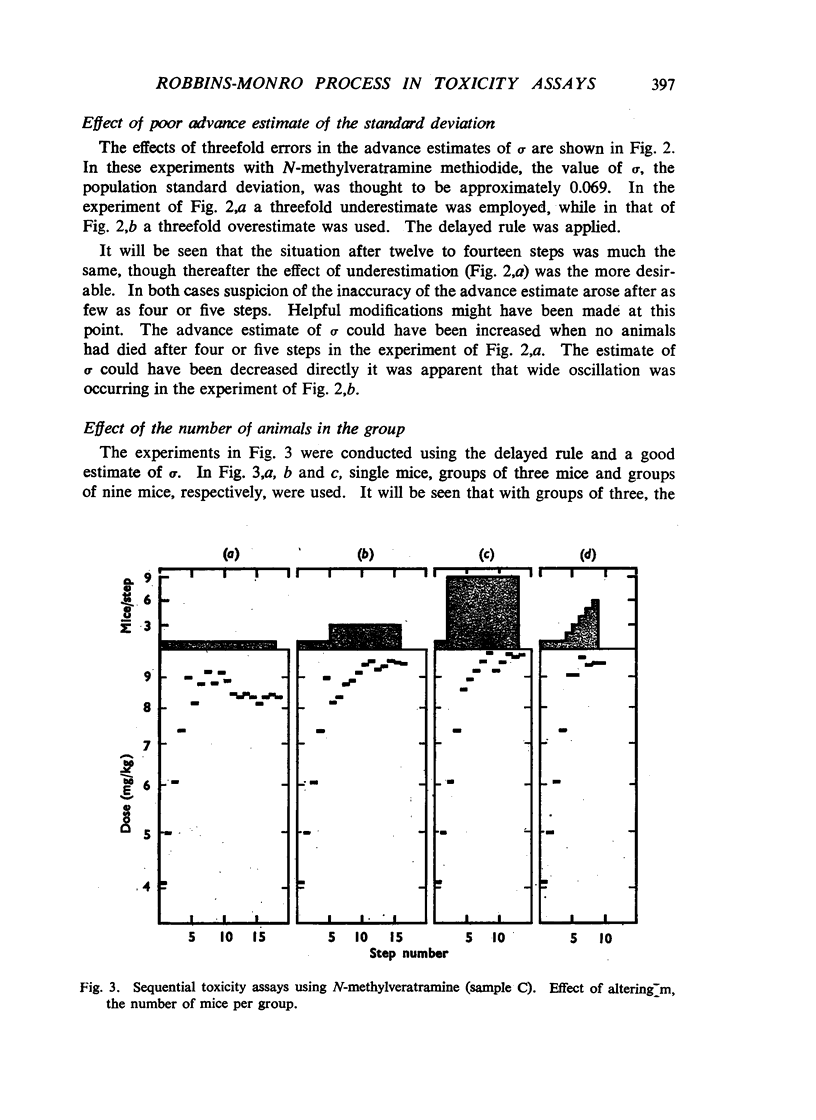





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- RUMKE C. L. The influence of atropine on the toxicity of thialbarbitone, thiopentone and pentobarbital (with an appendix on sequential toxicity testing). Arch Int Pharmacodyn Ther. 1959 Mar 1;119(1-2):10–19. [PubMed] [Google Scholar]


