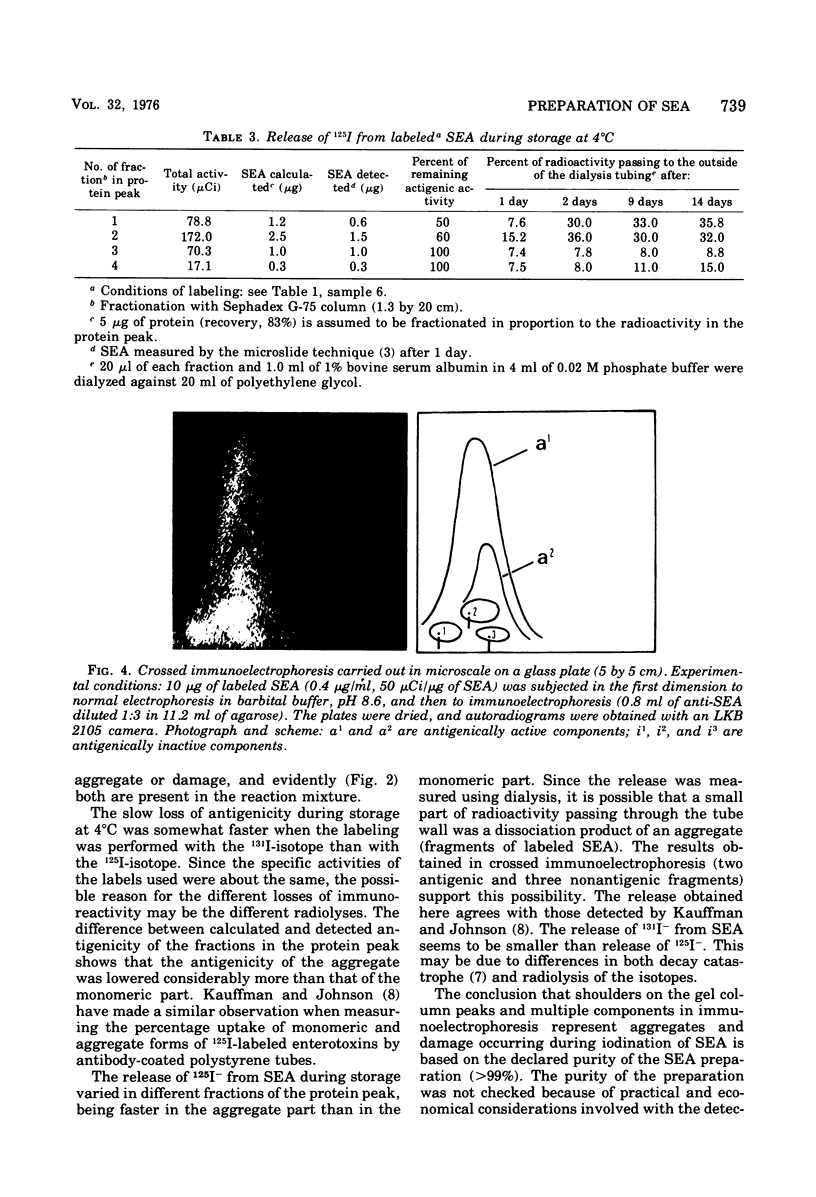
Abstract
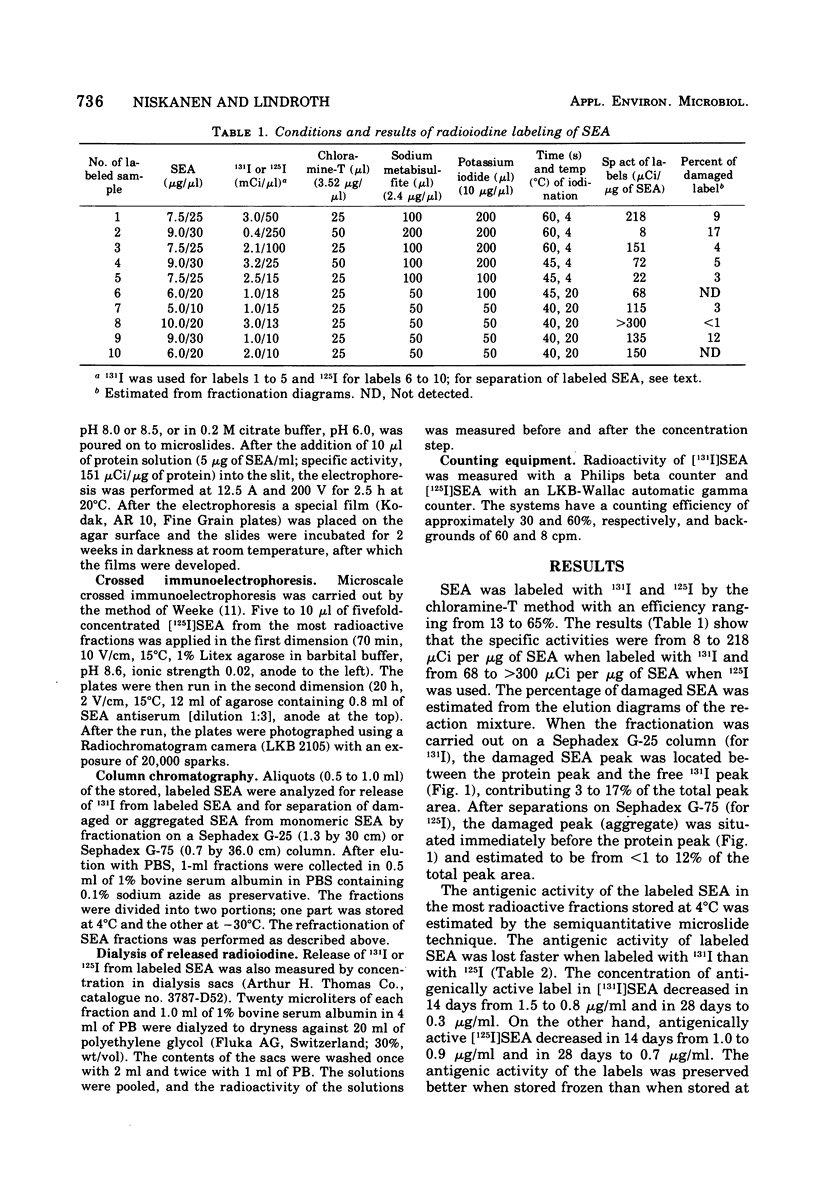
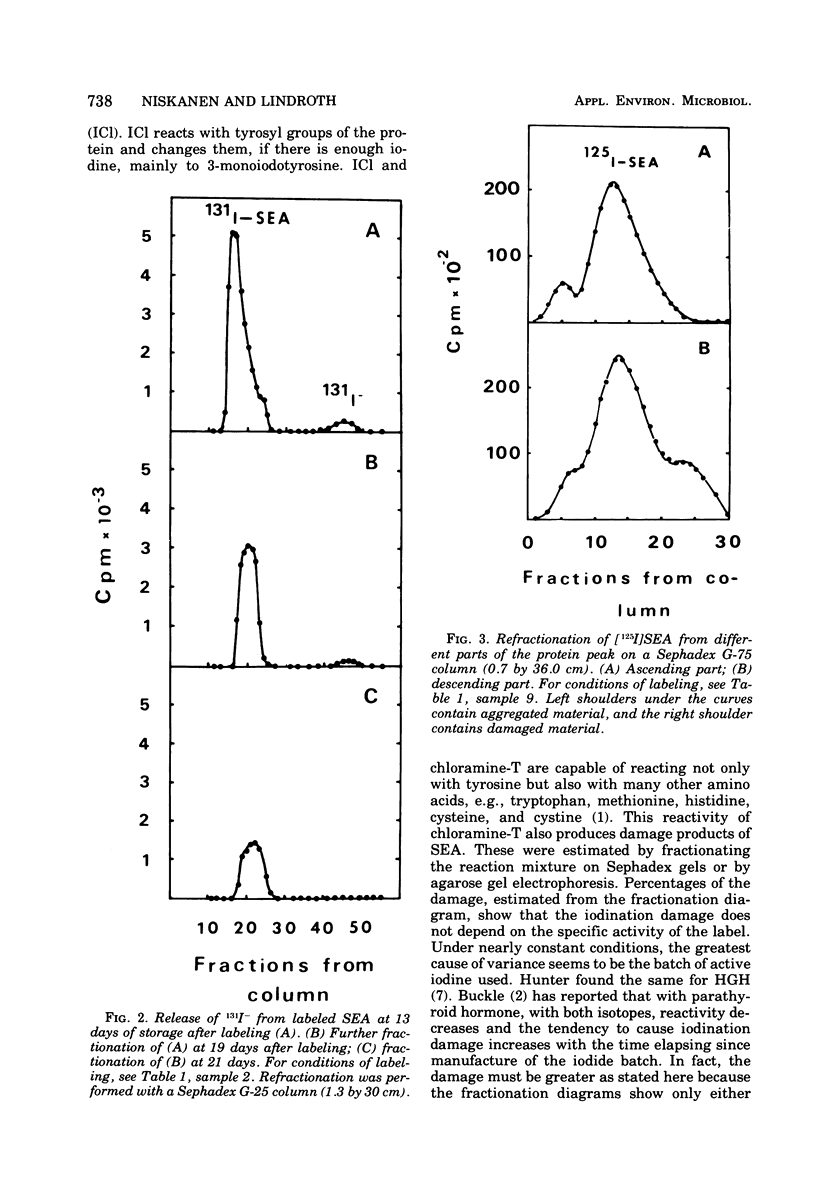
Staphylococcal enterotoxin A (SEA) was labeled by the chloramine-T method with 125I to a specific activity of 68 to 300 muCi per mug of SEA and with 131I to specific activity of 8 to 218 muCi per mug of SEA. SEA was partially damaged and aggregated during the labeling and storage. The damage seemed not to be greatly dependent on the specific activity of labeled entertoxin. Crossed immunoelectrophoresis showed two antigenically active and three inactive components in the ascending part of the labeled enterotoxin peak during fractionation by gel chromatography. During storage at 4 degrees C, the antigenic activity of label decreased faster when labeling had been with 131I than when with 125I. The antigenic activity of labeled SEA was lowered remarkably in the ascending part of the protein peak. Greatest release of radioiodine during storage was in the same part of protein peak. According to these results, the most suitable label for radioimmunoassay is obtained from the descending part of protein peak.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. M. Oxidation and oxidative cleavage of tryptophanyl peptide bonds during iodination. Biochem Biophys Res Commun. 1973 Sep 18;54(2):614–621. doi: 10.1016/0006-291x(73)91467-8. [DOI] [PubMed] [Google Scholar]
- Casman E. P., Bennett R. W., Dorsey A. E., Stone J. E. The micro-slide gel double diffusion test for the detection and assay of staphylococcal enterotoxins. Health Lab Sci. 1969 Oct;6(4):185–198. [PubMed] [Google Scholar]
- Collins W. S., Johnson A. D., Metzger J. F., Bennett R. W. Rapid solid-phase radioimmunoassay for staphylococcal enterotoxin A. Appl Microbiol. 1973 May;25(5):774–777. doi: 10.1128/am.25.5.774-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R. Standardization of the chloramine-T method of protein iodination. Proc Soc Exp Biol Med. 1970 Mar;133(3):989–992. doi: 10.3181/00379727-133-34611. [DOI] [PubMed] [Google Scholar]
- Kauffman P. E., Johnson H. M. Stability of 125I-labeled staphylococcal enterotoxins in solid-phase radioimmunoassay. Appl Microbiol. 1975 Jun;29(6):776–779. doi: 10.1128/am.29.6.776-779.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskanen A., Lindroth S. Comparison of different purification procedure for extraction of staphylococcal enterotoxin A from foods. Appl Environ Microbiol. 1976 Oct;32(4):455–464. doi: 10.1128/aem.32.4.455-464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robern H., Dighton M., Yano Y., Dickie N. Double-antibody radioimmunoassay for staphylococcal enterotoxin C2. Appl Microbiol. 1975 Oct;30(4):525–529. doi: 10.1128/am.30.4.525-529.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


