Abstract
Our aim was to conduct a prospective study to evaluate staging accuracy of a new coil concept for endoluminal magnetic resonance imaging (MRI) on ex vivo gastric carcinomas. Twenty-eight consecutive patients referred to surgery with a clinically proven primary gastric malignancy were included. Surgical specimens were examined with a foldable and self-expanding loop coil (8-cm diameter) at 1.5 Tesla immediately after total gastrectomy. T1- and T2-weighted and opposed-phase sequences (axial, frontal sections; 3- to 4-mm slice thickness) were acquired. Investigators blinded to any patient information analyzed signal intensity of normal gastric wall, gastric tumor, and lymph nodes. Findings were compared with histopathological staging. On surgical specimens, 2–5 gastric wall layers could be visualized. All gastric tumors (26 carcinomas, two lymphomas) were identified on endoluminal MR data (100%). Overall accuracy for T staging was 75% (18/24); sensitivity to detect serosal involvement was 80% and specificity 89%. N staging correlated in 58% (14/24) with histopathology (N+ versus N−). The endoluminal coil concept is feasible and applicable for an ex vivo setting. Endoluminal MR data provided sufficient detail for gastric wall layer differentiation, and therefore, identification of T stages in gastric carcinoma is possible. Further investigations in in vivo settings should explore the potential of our coil concept for endoluminal MR imaging.
Keywords: Stomach neoplasms, Magnetic resonance imaging, Coils, Neoplasm staging, Experimental study
Introduction
Despite the fact that the incidence of gastric cancer is declining in most Western countries, it remains the second leading cause of cancer mortality worldwide [1, 2]. Simultaneously, the incidence and prevalence of cancer arising from the gastric cardia has been increasing since the 1970s [3]. Adenocarcinoma of the esophagogastric junction can be classified according to Siewert et al. based on morphology and anatomical location [4]. Adequate surgical resection (R0) is the only potentially curative therapy for eligible patients with gastric carcinoma [4]. In the West, gastric cancer is often diagnosed at an advanced stage of disease not eligible for surgery. Fewer than 50% of patients undergo R0 resection [5, 6]. Recently, new concepts of multimodal treatment strategies for locally advanced gastric carcinoma have been investigated [7, 8]. Local treatment by endoscopic mucosal resection for early gastric carcinomas is currently being evaluated [9, 10]. Several phase II and III clinical trials for neoadjuvant chemotherapy in gastric carcinoma showed its feasibility and safety [11, 12]. Its purpose is to eliminate or delay systemic metastasis and reduce micrometastatic spread of disease. Another benefit is potential reduction of tumor volume in initially unresectable advanced tumor stages (downstaging), therefore increasing resectability rate. An adequate treatment strategy, especially in regard to the concept of neoadjuvant chemotherapy, requires precise clinical staging to depict relevant prognostic factors and identify resectable tumor stages. This also accounts for endoscopically treated early gastric cancers as well as for adenocarcinomas of the gastroesophageal (GE) junction since each type of tumor requires a different surgical approach. Thus, the exact knowledge of tumor morphology and tumor invasion into gastric wall is a crucial information for clinical staging.
Up to now, computed tomography (CT) and magnetic resonance imaging (MRI) have not provided the spatial resolution to fulfill this requirement with sufficient accuracy [1, 13–16]. Results for staging accuracy of endoscopic ultrasound (EUS) also seem to be improvable [17–19]. The main aspect of gastric wall differentiation is spatial resolution. By using endoluminal radiofrequency (RF) coils for MRI, image quality and spatial resolution can be enhanced. MRI provides superior soft tissue contrast, which makes it useful for tumor invasion detection; furthermore, information not obtainable by other imaging modalities is acquired. Various authors reported the use of endoluminal RF coils as a diagnostic and staging tool in gastrointestinal diseases and rectal and prostate cancer [20–27]. A major problem with endoluminal RF coils is placement close to the region of interest and depth of visualization. Our approach uses a foldable and afterward self-expanding loop coil design (8-cm diameter), which enhances spatial resolution and depth of visualization The aim of our study was to assess normal gastric wall architecture and signal intensity (SI) on endoluminal MRI as well as signal intensity and appearance of gastric carcinomas and their related lymph nodes. Subsequently, we used endoluminal MRI as staging modality on ex vivo gastric carcinomas. Findings were compared with histopathological staging.
Materials and methods
Patients
A prospective study was conducted on patients with gastric malignancies referred for total gastrectomy to the surgery department of our university hospital. They were included in the study if the lesion was identified by EUS or CT suggesting a gastric carcinoma or if histological workup of biopsies confirmed the diagnosis. The study protocol was approved by the institutional review board, and informed consent was acquired from each patient prior to surgery. We investigated 28 consecutive patients, 11 women and 17 men (age range 46–87, median 67 years). Tumors were located at the cardiac region in 11 cases, the fundal area in two, the corpus in six, and the antropyloric area in nine. Immediately after surgery, gastrectomy specimens were taken to the MR suite to conduct the examination within a time frame of 2–3 h (Fig. 1). Specimens where then sent to histopathology unchanged from the procedure.
Fig. 1.
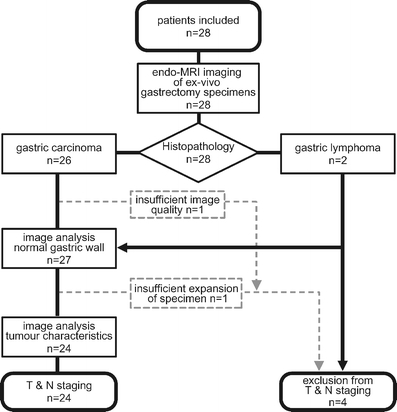
Flowchart of study design
Endoluminal RF coil
The endoluminal RF coil (patent issued 10/2005, German Patent and Trade Mark Office, No: 10127850.0–35, Grenacher et al.), developed in collaboration with Fraunhofer Institute for Biomedical Engineering (IBMT, St. Ingbert, Germany) consists of a foldable and self-expanding receiver loop (8-cm diameter). It is coated with a biocompatible material (silicone) to prevent direct contact of the wire with stomach tissue [29]. The flexible characteristics of the shape memory metal (nitinol) used allow passage through the instrument channel (13-mm diameter) of a specially designed MR-compatible endoscope. The MR-compatible endoscope was not used in this study and is described elsewhere, but it is mentioned in this context because RF coil design is reflected by the specifications of the endoscope. A nonferromagnetic tuning box connects the RF coil via a standard interface with a 1.5-Tesla MRI scanner (Symphony, Siemens, Erlangen, Germany), which was used in this study. Gastrectomy specimens were placed in a container with 3–4 l of 0.9% sodium chloride solution to ensure good contrast with gastric tissue and adequate expansion of gastric folds. The loop of the RF coil was placed on the mucosa side of the tumor. After the tumor was located with a scout sequence, images where acquired in axial and frontal sections of 3- to 4-mm thickness. Scanning was performed with T1- and T2-weighted and opposed-phase sequences (Table 1).
Table 1.
Imaging sequence parameters
| Sequence | Repetition time (TR) | Echo time (TE) | Acquisitions | Slice thickness (mm) | Matrix | Field of view (FOV) (mm) | Pixel size (mm) |
|---|---|---|---|---|---|---|---|
| T1-weighted TSE | 551 | 14 | 2 | 4 | 512x512 | 200 | 0.39x0.39 |
| T2-weighted TSE | 3,520 | 70 | 2 | 3 | 1024x1024 | 220 | 0.21x0.21 |
| T2-weighted GRE | 1,050 | 35 | 1 | 3 | 512x512 | 220 | 0.43x0.43 |
| T1-weighted GRE opposed phase | 187 | 7 | 4 | 3 | 256x265 | 220 | 0.86x0.86 |
TSE turbo spin echo, GRE gradient recalled echo
Image evaluation
Image data were read on certified diagnostic work stations independently by two board-certified radiologists with 10 and 12 years of experience, respectively, and one board-certified surgeon with 8 years of experience in endoscopic ultrasound on gastric carcinomas. Investigators were blinded to any clinical information as well as patient identification. After separate reading, a discussion was held about the quality of data and unique features of the case. Additionally, final agreement was achieved if disagreement on tumor stage had initially occurred. With respect to the unique imaging technique, the resolution and detail of image data a consensus staging was reasonable. Radiological and histopathological classification of the tumor was done according to the Tumor Node Metastisis (TNM) staging system of the Union Internationale Contre le Cancer (UICC) [30]. Determination of tumor stage was done as follows:
T1: tumor invasion of the lamina propria of mucosa or invasion of submucosa, wall thickening, and signal intensity changes confined to these layers.
T2: tumor invasion extended to the muscularis propria, and additional thickening through gastric layers with signal intensity changes showing either a homogeneous or inhomogeneous lesion without serosal abnormalities. If invasion extended beyond muscularis propria into an adjacent fat tissue plane without serosal infiltration, it was considered as T2.
T3: tumor invasion of mucosa, submucosa, and muscularis propria, with infiltration of the serosa or changes in signal intensity presenting with micronodular strands as growth into extraserosal fat tissue.
T4: tumor invasion into adjacent organs or structures clearly presenting as contiguous tumor extension or a mass with similar signal intensity as the gastric tumor.
N staging was done counting the lymph nodes detectable on MRI, regarding them as pathologic due to their signal intensity. Since gastrectomy samples varied in lymph node content due to surgical technique and extension of the gastrectomy not comparable to an in vivo situation, N factor was graded into N+ for positive findings of lymph nodes and into N− for absence of lymph nodes. In each case, normal gastric wall features were assessed. Signal intensities of the mucosa, submucosa, muscularis propria, and serosa or subserosa were recorded, as well as the amount of layers in which the normal gastric wall could be differentiated into. A score system from 1 to 5 (1=poor; 5=very good) was used for several study items to weigh the power of their findings. Each sequence, for example, was rated for image quality on a range from 1 to 5, representing insufficient to very good. Signal intensity and morphology of the gastric tumors and demarcation to normal gastric wall were used to describe characteristics of tumor presentation. The lesion was identified by irregular architecture of gastric wall, abnormal thickening, and change in signal intensity of gastric wall layers, which usually present with sharp and clear demarcation into three to five different layers with distinct signal intensities and thickness. Tumor size was not measured, as it does not influence T staging or provide further information. Histopathology was considered the gold standard, and radiological data where compared with its results.
All data are presented as absolute numbers and relatively as percentages. Concordance of histopathological results and radiological findings are reported as overall accuracy. Sensitivity, specificity, and accuracy for T factor, N+/− factor are provided. Detection rate of serous membrane invasion or the differentiation between T2 and T3 stages was assessed, as it defines advanced stages of disease. Ninety-five percent confidence intervals (CI) are given if statistically applicable.
Results
The setup of our study proved to be feasible, as shown elsewhere [29]. Two cases were not included because of insufficient image quality. Histopathology found 26 carcinomas (15 adenocarcinomas, nine signet cell carcinomas, two mixed type carcinomas) and two gastric lymphomas. Four tumors were classified as pT1, 15 as pT2, 3 as pT3, and 2 as pT4. Gastric lymphomas were excluded from the study although they where detected as such in both cases (100%).
Gastric tumor staging
In 24 (100%) cases, the tumor was identified. Initially 46% (11/24) of T stages were staged correctly; 1/4 of pT1 tumors, 7/15 of pT2 tumors, 2/3 of pT3 tumors, and 1/2 of pT4 tumors were classified correctly (Table 2). Interpretation of image data was done without knowledge of tumor location in respect to the gastric region, such as cardia, fundus, corpus, or antrum, as it would be possible on an in vivo setting such as endoscopy or CT. In a second reading session, the interpreters were informed about the gastric region of the primary tumor for each case. In knowledge of gastric region, six, first as T3 classified carcinomas of the cardia, were correctly staged as pT2 and one, first as T3 classified tumor (cardia), was correctly identified as pT4 (Table 3). This gives an overall accuracy of 75% (18/24)± –17.32 (CI) (Table 4). The mean score for T-factor staging was 3.54 (range 2–5). Sensitivity, specificity, and accuracy for detecting serosal involvement was 80%±16.00, 89%±12.27, and 88%±13.23, with a mean score of 4.5 (range 3–5; Table 5). Overstaging occurred in 4/24, three pT1 tumors were classified as T2 or T3 stages, and one pT2 tumor was identified as T3 stage (Table 6). In this case, the tumor replaced all gastric wall layers and invaded perigastric fat tissue but did not infiltrate the serous membrane. Insufficient separation of gastric wall layers and the presence of abnormal signal intensities most likely desmoplastic reactions or peritumor inflammation were identified retrospectively as reasons for overstaging of pT1 tumors. Two tumors (pT2, pT3) were understaged as T1 and T2 stage. One case was a diffuse type pT2 adenocarcinoma with disseminated infiltration into the muscularis propria, which is not visualized and thus presented as T1 stage.
Table 2.
Comparison of histologic with endoluminal findings without knowledge of tumor region
| Endoluminal magnetic resonance imaging (MRI) findings | |||||
|---|---|---|---|---|---|
| Histologic findings | T1 | T2 | T3 | T4 | Total (n=24) |
| T1 | 1 | 2 | 1 | – | 4 |
| T2 | 1 | 7 | 7 | – | 15 |
| T3 | – | 1 | 2 | – | 3 |
| T4 | – | – | 1 | 1 | 2 |
Table 3.
Comparison of histologic findings with endoluminal findings acquired with knowledge of tumor region
| Endoluminal magnetic resonance imaging (MRI) findings | |||||
|---|---|---|---|---|---|
| Histologic findings | T1 | T2 | T3 | T4 | Total (n=24) |
| T1 | 1 | 2 | 1 | — | 4 |
| T2 | 1 | 13 | 1 | — | 15 |
| T3 | – | 1 | 2 | – | 3 |
| T4 | – | – | – | 2 | 2 |
Table 4.
Sensitivity, specificity, and accuracy for each T stage; overstaging and understaging rates
| T1 | T2 | T3 | T4 | Overall | |
|---|---|---|---|---|---|
| Sensitivity | 1/4 | 13/15 | 2/3 | 2/2 | – |
| Specificity | 19/20 | 6/9 | 19/21 | 22/22 | – |
| Accuracy | 20/24 | 19/24 | 21/ 24 | 24/24 | 18/24 |
| Overstaging | 3/4 | 1/15 | – | – | 4/24 |
| Understaging | – | 1/15 | 1/3 | – | 2/24 |
Table 5.
Results for serosal invasion detection
| Evaluation of serosal invasion | |
|---|---|
| Sensitivity | 4/5 |
| Specificity | 17/19 |
| Accuracy | 21/24 |
| False positive | 2 |
Table 6.
Signal intensities of gastric wall layers on T1-weighted, T2-weighted, and opposed-phase imaging from inside to outside
| T1 imaging (n=21)a | T2 imaging (n=27)a | Opposed phase imaging (n=3)a | |
|---|---|---|---|
| Two layers (n=7)b | High | – | – |
| Intermediate | |||
| Three layers (n=33)b | High | Intermediate | High |
| Low | High | Low | |
| Intermediate | Intermediate | High | |
| Four layers (n=10)b | High | Intermediate | – |
| Low | Low | ||
| Intermediate | Intermediate | ||
| Low | Low | ||
| five layers (n=1)* | – | Intermediate | – |
| High | |||
| Intermediate | |||
| Low | |||
| Intermediate |
aNumber of sequences analyzed
bNumber of sequences with n layers
Nodal involvement
Lymph node detection resulted in N+ for detected lymph nodes in 17 cases with N− in 7 cases. Sensitivity for detecting N+ was 71%±18.22, specificity 29%±18.07, and accuracy 58%±19.72. This reflects the limitation of the study design. Ex vivo gastrectomy specimens do not provide the same quantity of lymph nodes as pathological workup. The mean score rating N factor staging was 4.4 (range 2–5).
Image analysis
Separation of gastric wall layers ranged from a minimum of two visible layers to five distinct visible layers (mean 3.10) in 51 analyzed sequences: 21 T1-weighted and 27 T2-weighted sequences and three opposed-phase sequences (Table 6). T2-weighted sequences depicted an average of 3.37 gastric wall layers; T1-weighted sequences 2.76. Mean score for image quality of the sequences used was 3.88 (range 2–5), with 4.11 for T2- and 3.62 for T1-weighted sequences. Image analysis revealed that in four out of six incorrectly staged T factors, tumors were described as inhomogeneous or diffuse concerning demarcation to normal gastric wall. This may be one reason for misinterpretation of T factor. Signal intensity of the mucosa for T1-weighted sequences was mostly classified as hyperintense; for T2-weighted, it was intermediate. Submucosa, muscularis propria, and serosa or subserosa presented with hypointense, intermediate, hypointense SI for T1-weighted; hyperintense, intermediate, hypointense for T2-weighted sequences (Table 6). Lymph node and tumor appearance correlated in 86%±9.9 (44/51 sequences), with intermediate SI for T1- and T2-weighted sequences. Out of eight sequences without correlation in lymph node and tumor SI, two sequences showed a homogeneous tumor in T1-weighted and an inhomogeneous lesion in T2-weighted images (Fig. 3). In three opposed-phase sequences, lymph nodes (hypointense) showed different signal intensity than the primary tumor (intermediate). T2-weighted sequences (Table 4) visualized more gastric wall layers and provided the interpreter with a better demarcation of tumor volume from normal gastric wall than did T1. T1-weighted sequences showed a better delineation and contrast of lymph nodes in adjacent structures (Fig. 2).
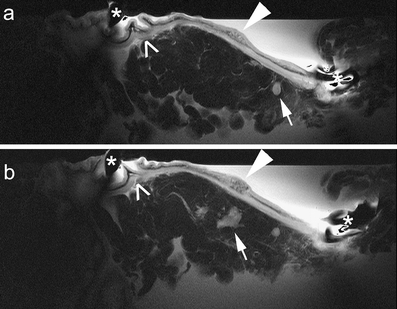
Fig. 3a, b.
T2 signet cell tumor of the cardia region (diffuse type of Lauren classification, white arrowhead) on T1-weighted (a) and T2-weighted imaging (b). The extent of tumor mass and the diffuse infiltration into gastric wall is better visualized on T2-weighted images due to the mucinous character of the tumor (black arrowheads). The tumor appears more homogeneous on T1-weigthed images (a). (*) marks the position of the receiver coil
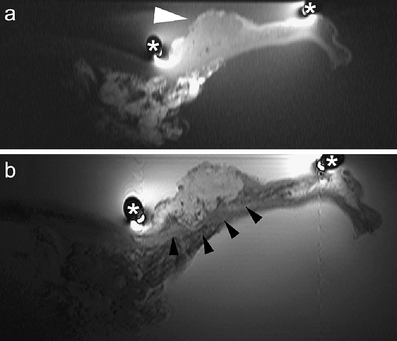
Fig. 2a, b.
Two consecutive T2-weighted images (a, b) of a T1 early gastric carcinoma (white arrowheads), well differentiated (intestinal type of Lauren classification), located at the subcardial region. Lymph nodes in adjacent fat tissue with a high signal intensity (white arrows) are visualized. Morphology of normal gastric wall is pointed out by open arrows. (*) marks the position of the receiver coil
Discussion
Endoluminal MRI has been used by various groups to asses its ability for locoregional imaging of esophageal and gastrointestinal disease. The first group [20, 31] to introduce an MR endoscope reported promising results for the diagnosis of gastrointestinal diseases and pointed out limitations of their design [20, 31]. Image quality of endoluminal MRI was not as clear as those acquired by EUS, and their success rate of obtaining clear images of the stomach was low (58%) [20]. Although their study achieved 89% for T-staging accuracy, its patient collective consisted mainly of advanced gastric carcinomas (T3, T4). Other authors reported the use of an MR endoscope with a similar design [21, 28, 32], sharing the incorporation of the RF coil with 10×30 and 10×47 mm into the tip of the MR endoscope. This is a useful design for imaging at the esophagus, where both groups showed good results in staging esophageal cancer [20, 28]. The small cylindrical RF coil design implemented in the tip of the MR endoscope may be a reason for problems in obtaining sufficient images in the stomach. Our approach using an insertable and self-expandable RF coil (8-cm diameter), independent from the MR endoscope, seems to be more suitable for imaging of the stomach by providing enhanced depth of visualization. In 93%, sufficient image data was obtained. Detection of resectable lesions can only be accomplished by exact knowledge of tumor invasion in relation to gastric wall layers. New multimodal treatment strategies require the information of tumor stage, as it defines enrollment into neoadjuvant therapy approach. Clinical staging based on imaging studies can provide important information for adequate therapy decision if they fulfill this requirement. Gastric wall differentiation and identification is essential for accurate staging of gastric malignancies.
Our approach can depict between two to five gastric wall layers and therefore deliver sufficient detail as basis for tumor invasion detection. Signal intensities of gastric layers assessed in our study correspond mainly to those reported previously by other authors [20, 33, 34]. Visualization of the serosa layer remains a problem in all published studies with MRI or endoluminal MRI on gastric wall. Some authors investigated ex vivo gastrectomy specimen with a 1.0-Tesla MRI device and were able to differentiate up to five different gastric layers, but visualization of the serosa or subserosa was not achieved [35]. One reported study used a 2.4-Tesla MRI system and failed, as did another study with a 4.7-Tesla MRI system, to visualize serosa or subserosa as a distinct layer [34, 36]. One work group reported possible identification of serosa or subserosa on gastrectomy specimens after fixation with formalin [33]. With the use of a 1.5-Tesla MRI device and a 4-cm-diameter loop coil, muscularis propria and serosa or subserosa showed isointense signal intensities but were outlined by subserosal fat tissue with contrasting signal intensity. The results were confirmed by another work group using a similar setup [37]. Identification of the serosa is necessary to securely differentiate between T2- and T3-stage gastric carcinoma. Although a detection rate of 88% for serosal involvement was achieved in this study, serosa as a single gastric wall layer was not clearly visualized. In seven cases with four to five visible gastric layers, a thin outer layer on T2-weighted sequences was detected and could possibly be identified as serosa or subserosa with subserosal fat tissue. This finding was inconsistent and varied in different T-stage carcinomas. This may be because of isointense signal intensity of muscularis mucosae and serosa or subserosa or the fact that the thickness of the serosa layer is not sufficient for secure identification [38]. It is not certain whether tumor invasion into or close to the serosa or subserosa layer changes its signal intensity presentation. Indirect detection by subserosal fat tissue seems possible, but in our experience, there is some variance in the thickness of subserosal fat tissue in gastric carcinoma specimens. One reason for this could be tumor cachexia.
An interesting learning point is reflected by the results of T staging. Knowledge of the gastric region where the tumor is located is important since T staging of the stomach depends on the gastric region. Tumors of the cardia and part of the fundus region where a serosal layer is missing are considered T2 whereas they would be T3 in other gastric regions. The study setup was intended to be as close as possible to a future in vivo setting with an MR endoscope, but tumor location and gastric region cannot be read on image data from gastrectomy specimens. An in vivo setting would enable investigators to acquire this information. After providing the tumors' gastric region, T staging accuracy improved to 75%. Thus, the gastric region of tumor location is an important fact for adequate staging of tumor invasion. While this study used an ex vivo model of gastric carcinomas and the amount of patients included could be higher, the entire range of T factors for gastric carcinomas is covered. Another limitation of this study is the missing congruence of lymph nodes present in the gastrectomy specimens due to surgical technique to those available for histopathology. This is reflected by N-factor staging results. An in vivo setting should be more accurate but poses new problems, such as motion artefacts due to patient or endoscope movement. Modern rapid MRI sequences and spasmolytic agents may be able to overcome these obstacles in an in vivo setting, but this is a goal for future studies. It is important to point out pitfalls of this imaging technique, as overstaging is a problem in many imaging modalities. Peritumor inflammation, micronodular affection of the serosal membrane, and diffuse-type gastric carcinoma were retrospectively identified as reasons for misinterpretation of tumor invasion. Overstaging occurred in 17%, with three T1 tumors not correctly classified. This is encouraging since all T1 tumors were detected as tumors but in two cases were classified as T2 tumors and one as T3. Only one incorrectly classified T2 tumor contributed to overstaging. This indicates that overstaging was not a problem of differentiation between T2 and T3 stages of disease.
It is necessary to focus on T1 carcinomas, as experience with T1 carcinomas in imaging modalities in Western countries is low due to the fact that most gastric cancers are diagnosed at an advanced stage. It is important to learn more about signal intensity and morphologic presentation of early gastric carcinomas on endoluminal MRI to improve detection rate. Endoluminal MR imaging provides enough detail to visualize a T1 tumor (Fig. 2). Endoscopic mucosal resection as one treatment option for early gastric carcinoma requires an imaging method that is able to visualize and identify these stages securely and provide information that is observer independent. Further studies should include a larger quantity of early stages of gastric carcinomas to analyze accuracy potential of endoluminal MRI. MR images provide information not obtainable by other imaging modalities. A gastric tumor can present with different appearances on T1-weighted imaging than on a T2-weighted imaging (Fig. 3). Depth of visualization of a method is essential for staging a gastric tumor, assessing its depth of invasion into gastric wall and into adjacent organs and structures, as well as depicting involved lymph nodes. Our image data can provide detailed information about gastric wall invasion as well as organ invasion (Figs. 2 and 4).
Fig. 4a, b.
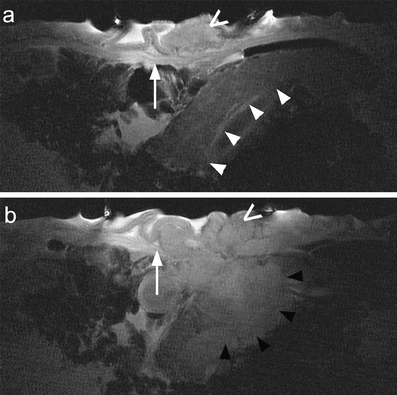
T4 gastric tumor (intestinal type of Lauren classification, open arrows) on T2-weighted images of the same plane. Transition of normal gastric wall into tumor mass (white arrows). Pancreas (white arrowheads) adjacent to the gastric wall demonstrates depth of visualization (a). Tumor invasion into pancreas (black arrowheads) (b)
In this study, we have shown that our concept is functional and feasible for MRI of the stomach, and staging results comparable with other imaging modalities were achieved. Endoluminal MRI in combination with conventional MRI could assess local and regional imaging in one session, giving accurate information about local staging and metastatic spread of disease. Secondary contrast media should be evaluated for its use in improving gastric wall layer discrimination and identification as well as tumor demarcation. Additional studies are needed to assess the clinical feasibility of this coil concept in vivo for imaging of the upper gastrointestinal tract. High-resolution imaging of the pancreas could be another possible application. Today, most tumors of the stomach and esophagus are staged by conventional methods, such as endoscopy, EUS, or CT. Future prospects of endoscopic MRI may be limited to certain tumor entities, such as early or advanced carcinomas, but it could be able to answer additional specific clinical questions for relevant therapy decisions.
Summary Endoluminal MRI with the coil concept is feasible and applicable. T staging of ex vivo gastric carcinomas is possible. Results for T-factor staging are preliminary but promising, as they reflect the first experience with this technique. Image quality and resolution obtained with the endoluminal RF coil are convincing for future use in gastrointestinal imaging. Limitations of this study include missing congruence in quantity of lymph nodes for N staging, a limited amount of some T-stage gastric carcinomas, and the ex vivo setting. Taking the limitations into account, the coil design should be investigated in an in vivo setting after assessing safety aspects to evaluate the prospects given by the ex vivo results. The clinical potential may be limited by providing a staging tool for certain tumor entities. With more experience in endoluminal MRI and interpretation of endoluminal image data, its use could be extended toward other fields in gastrointestinal imaging.
References
- 1.Hohenberger P, Gretschel S (2003) Gastric cancer. Lancet 362(9380):305–315 [DOI] [PubMed]
- 2.Crew KD, Neugut AI (2004) Epidemiology of upper gastrointestinal malignancies. Semin Oncol 31(4):450–464 [DOI] [PubMed]
- 3.Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA (2000) Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol 29(4):645–654 [DOI] [PubMed]
- 4.Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85(11):1457–1459 [DOI] [PubMed]
- 5.Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Jr., Winchester D, Osteen R (1993) Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 218(5):583–592 [DOI] [PMC free article] [PubMed]
- 6.Adashek K, Sanger J, Longmire WP Jr (1979) Cancer of the stomach. Review of consecutive ten year intervals. Ann Surg 189(1):6–10 [DOI] [PMC free article] [PubMed]
- 7.Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, Feig B, Myerson R, Nivers R, Cohen DS, Gunderson LL (2004) Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol 22(14):2774–2780 [DOI] [PubMed]
- 8.Janunger KG, Hafstrom L, Nygren P, Glimelius B (2001) A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol 40(2–3):309–326 [DOI] [PubMed]
- 9.Abe N, Watanabe T, Sugiyama M, Yanagida O, Masaki T, Mori T, Atomi Y (2004) Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg 188(2):181–184 [DOI] [PubMed]
- 10.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48(2):225–259 [DOI] [PMC free article] [PubMed]
- 11.Allum WH, Cunningham DC, Weeden S (2003) On behalf of the NCRI Upper GI Clinical Study Group. Perioperative chemotherapy in operable gastric and lower oesophageal cancer. A randomised controlled trial (the MAGIC trial ISRCTN 93793971). Proc Am Soc Clin Oncol 998(22):249a, (abstract)
- 12.Crookes P, Leichman CG, Leichman L, Tan M, Laine L, Stain S, Baranda J, Casagrande Y, Groshen S, Silberman H (1997) Systemic chemotherapy for gastric carcinoma followed by postoperative intraperitoneal therapy: a final report. Cancer 79(9):1767–1775 [DOI] [PubMed]
- 13.Abdalla EK, Pisters PW (2004) Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol 31(4):513–529 [DOI] [PubMed]
- 14.Dux M, Richter GM, Hansmann J, Kuntz C, Kauffmann GW (1999) Helical hydro-CT for diagnosis and staging of gastric carcinoma. J Comput Assist Tomogr 23(6):913-922 [DOI] [PubMed]
- 15.Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, Dieckmann C, Schoder V, Adam G (2004) Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology 230(2):465–471 [DOI] [PubMed]
- 16.Davies J, Chalmers AG, Sue Ling HM, May J, Miller GV, Martin IG, Johnston D (1997) Spiral computed tomography and operative staging of gastric carcinoma: a comparison with histopathological staging. Gut 41(3):314–319 [DOI] [PMC free article] [PubMed]
- 17.Bosing N, Schumacher B, Frieling T, Ohmann C, Jungblut R, Lubke H, Bohner H, Verreet P, Roher HD (2003) Endoscopic ultrasound in routine clinical practice for staging adenocarcinomas of the stomach and distal esophagus. Chirurg 74(3):214–221; discussion 222–223 [DOI] [PubMed]
- 18.Wang JY, Hsieh JS, Huang YS, Huang CJ, Hou MF, Huang TJ (1998) Endoscopic ultrasonography for preoperative locoregional staging and assessment of resectability in gastric cancer. Clin Imaging 22(5):355–359 [DOI] [PubMed]
- 19.Meining A, Dittler HJ, Wolf A, Lorenz R, Schusdziarra V, Siewert JR, Classen M, Hofler H, Rosch T (2002) You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut 50(5):599–603 [DOI] [PMC free article] [PubMed]
- 20.Inui K, Nakazawa S, Yoshino J, Yamao K, Yamachika H, Wakabayashi T, Kanemaki N, Hidano H (1995) Endoscopic MRI: preliminary results of a new technique for visualization and staging of gastrointestinal tumors. Endoscopy 27(7):480–485 [DOI] [PubMed]
- 21.Feldman DR, Kulling DP, Hawes RH, Kay CL, Muckenfuss VR, Cotton PB, Bohning DE, Young JW (1997) MR endoscopy: preliminary experience in human trials. Radiology 202(3):868–870 [DOI] [PubMed]
- 22.Dave UR, Williams AD, Wilson JA, Amin Z, Gilderdale DJ, Larkman DJ, Thursz MR, Taylor-Robinson SD, deSouza NM (2004) Esophageal cancer staging with endoscopic MR imaging: pilot study. Radiology 230(1):281–286 [DOI] [PubMed]
- 23.deSouza NM, Kmiot WA, Puni R, Hall AS, Burl M, Bartram CI, Bydder GM (1995) High resolution magnetic resonance imaging of the anal sphincter using an internal coil. Gut 37(2):284–287 [DOI] [PMC free article] [PubMed]
- 24.Stoker J, van Velthuysen ML, van Overhagen H, van Kempen D, Tilanus HW, Lameris JS (1999) Esophageal carcinoma. Ex vivo endoluminal magnetic resonance imaging. Invest Radiol 34(1):58–64 [DOI] [PubMed]
- 25.Minoli G (1996) Endoscopic MRI: preliminary results of a new technique for visualization and staging of gastrointestinal tumours. Gastrointest Endosc 44(5):639–640 [DOI] [PubMed]
- 26.Maldjian C, Smith R, Kilger A, Schnall M, Ginsberg G, Kochman M (2000) Endorectal surface coil MR imaging as a staging technique for rectal carcinoma: a comparison study to rectal endosonography. Abdom Imaging 25(1):75–80 [DOI] [PubMed]
- 27.D’Amico AV, Schnall M, Whittington R, Malkowicz SB, Schultz D, Tomaszewski JE, Wein A (1998) Endorectal coil magnetic resonance imaging identifies locally advanced prostate cancer in select patients with clinically localized disease. Urology 51(3):449–454 [DOI] [PubMed]
- 28.Kulling D, Feldman DR, Kay CL, Hoffman BJ, Reed CE, Young JW, Hawes RH (1998) Local staging of esophageal cancer using endoscopic magnetic resonance imaging: prospective comparison with endoscopic ultrasound. Endoscopy 30(9):745–749 [DOI] [PubMed]
- 29.Grenacher L, Heye T, Kuntz C, Palmowski M, Autschbach F, Manz B, Benecke M, Volke F, Kauffmann GW, Dux M (2005) Experimental testing of a new coil design for endoluminal MRI applied to the pig stomach. Rofo 177(7):986–991 [DOI] [PubMed]
- 30.Sobin LH, Wittekind C (2002) In: International Union Against Cancer (UICC) (eds) TNM Classification of Malignant Tumours, 6th edn. Wiley, New York
- 31.Inui K, Nakazawa S, Yoshino J, Ukai H. (1998) Endoscopic MRI. Pancreas 16(3):413–417 [DOI] [PubMed]
- 32.Kulling D, Bohning DE, Kay CL, Feldman DR, Cotton PB, Hawes RH (1997) Histological correlates to pig gastrointestinal wall layers imaged in vitro with the magnetic resonance endoscope. Gastroenterology 112(5):1568–1574 [DOI] [PubMed]
- 33.Yamada I, Saito N, Takeshita K, Yoshino N, Tetsumura A, Kumagai J, Shibuya H (2001) Early gastric carcinoma: evaluation with high-spatial-resolution MR imaging in vitro. Radiology 220(1):115–121 [DOI] [PubMed]
- 34.Lubienski A, Grenacher L, Reith W, Schipp A, Mechtersheimer G, Dux M (2002) [MR imaging of gastric wall layers in vitro: correlation to the histologic wall structure]. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 174(4):490–494 [DOI] [PubMed]
- 35.Dux M, Roeren T, Kuntz C, Schipp A, Scheller D, Mechtersheimer G, Kauffmann GW (1997) MRI for staging of gastric carcinoma: first results of an experimental prospective study. J Comput Assist Tomogr 21(1):66–72 [DOI] [PubMed]
- 36.Auh YH, Lim TH, Lee DH, Kim YH, Lee MG, Cho KS, Mun CW, Lee I (1994) In vitro MR imaging of the resected stomach with a 4.7-T superconducting magnet. Radiology 191(1):129–134 [DOI] [PubMed]
- 37.Sato C, Naganawa S, Kumada H, Miura S, Ishigaki T (2004) MR imaging of gastric cancer in vitro: accuracy of invasion depth diagnosis. Eur Radiol 14(9):1543–1549 [DOI] [PubMed]
- 38.Bolondi L, Caletti G, Casanova P, Villanacci V, Grigioni W, Labo G. (1986) Problems and variations in the interpretation of the ultrasound feature of the normal upper and lower GI tract wall. Scand J Gastroenterol Suppl 123:16–26 [DOI] [PubMed]


