Abstract
In this work, we analysed the effect of growth factors on in vitro cell proliferation and collagens synthesis by fibroblasts cultured for 72 h on different substrates (silicon sheet with or without 1% gelatin, and glass as control surface) for ligament tissue engineering. A human fibroblast cell line (CRL-2703) was used. The synthesis of type I and type III collagens were evaluated qualitatively and quantitatively by RT-PCR and confocal microscopy, respectively. Cell proliferation was evaluated by two methods: (1) MTT assay (2) cell cycle analysis. It was found that PDGF-AB stimulate the proliferation of fibroblast cultured on gelatin coated silicon sheet in dose dependant manner with a maximum effect at 10 ng ml−1. The exogenous TGF-β1 induced the expression of type I and type III collagens in a dose and substrate-dependant manner. We deduce from this work that biochemical conditions and substrates have an important impact for optimisation of the tissue neo synthesis.
Keywords: Cell proliferation, Collagens synthesis, Fibroblasts, Growth factors, Substrates
INTRODUCTION
Tissue engineering is a multidisciplinary field at the crossroads of biochemistry, mechanics, engineering and materials science to develop substitutes for replacing injured or diseased tissues.14,15,25,48 For all applications, it is necessary to take into account of several points: cell source, growth factors, mechanical forces, substrates, and the behaviour of the cells on the substrate.
Substrates or scaffolds are defined as the matrix that provides a suitable environment for cell proliferation and differentiation. Some scaffolds have been prepared from natural and synthetic materials, such as collagen, poly (glycolic acid) (PGA), and copolymers of l-lactic acid and є-caprolactone. It is necessary to improve the physical and chemical nature of scaffold for the superior induction of tissue regeneration. However, the chemical composition of the scaffold can have great influence on its biocompatibility.5,38 The source, solubility, degree of cross linking, physical form of the protein coating substrates, and the substrates itself can influence the cell functions like proliferation and metabolic activity.5
Cells and materials are two essential components in ligament tissue engineering. Materials could interfere with cells adhesion, proliferation, and differentiation. Currently, there are two common approaches to modify the materials to improve their biocompatibility, coating with biocompatible materials, or surface modification. As nearly all interaction between cells and artificial surfaces are mediated by a layer of absorbed protein, many proteins have been coated on the surface of target materials to promote adhesion, (i.e. fibrinogen, collagen, hyaluronan,...).13 Protein coated substrates are provided as a two dimensional substrates. Moreover, it has been shown that fibroblast attachment, morphology, proliferation, and collagen synthesis varied from different culture substrates and the type of the fibroblast obtained from different organs/tissue.9
An appropriate scaffold for ligament regeneration must be able to provide mechanical strength to initially withstand the in vivo force and degrade safely at an appropriate rate in vivo.39In vitro studies, a planar artificial substrate (i.e. silicon membrane) is usually used for growing cells and subjected them to mechanical loading.
Furnishing scaffolds with biochemical factors are also promising. Many biochemical factors including growth factors could promote tissue regeneration and/or healing processes.13
Growth factors are known to play an important role in the healing response of connective tissues.27 Several reports indicate the significance of growth factors in the intrinsic healing process of injured ligaments.24,32 Many growth factors affect the tendon and ligament healing process after injury by stimulating extra-cellular matrix (ECM) synthesis and cell proliferation.3,17,21,47 It has been shown that exogenous Transforming Growth Factors β1 (TGF-β1) increased the collagen synthesis of human anterior cruciate ligament cells and human ligamentum flavum.22,35 Platelet Derived Growth Factor-AB (PDGF-AB), one of the major serum growth factor, is known to enhance cell proliferation and healing responses,26,46 as well as to increase rupture force, stiffness and breaking energy of the tissue.31
The definition of the optimal condition of culture, such as coating of the substrate, synergistic effect of the substrate and growth factors are required for ligament tissue engineering.
In order to define the optimal concentration of growth factors and the evolution of cells culture on silicon membrane, which will be subjected to cyclic stretch for later mechanical study, the objective of the present work was to investigate the influences of individual growth factors and substrates on the cell proliferation and ECM synthesis. In this work, we studied the cell viability, proliferation, collagen production of fibroblast seeded on the different substrates and the effect of PDGF-AB on the cell proliferation and TGF-β on the ECM synthesis to allow the optimisation of culture conditions, which could be used for in vitro reconstruction of ligament/tendon tissues.
MATERIALS AND METHODS
Cells and Culture Conditions
Human skin fibroblast cell line (CRL-2703) was purchased from American Type Culture Collection (ATCC, No. 2584882, Manassas, VA). The cells were placed in 75 cm2 plastic culture flask with Dulbecco’s modified Eagle’s medium/Nutrient F-12 (GibcoBRL, Life Technologie, France) containing 10% decomplemented foetal bovine serum (FBS) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 4 mM l-glutamine, 2,5 μg/ml fungizone, 1% non-essential amino acids (GibcoBRL, Life Technologie, France), 150 μg/ml l-ascorbic acid, 1.5 g/l NaHCO3 (Sigma, France) and were incubated at 37°C [air (95%) and CO2 (5%)]. The medium was changed every 2 days. At confluence, cells were detached from the culture flask with 0.125% trypsin–EDTA mixture (Sigma, France) for 10 min at 37°C, and then resuspended in complete medium until confluence.
Substrate Surface Treatment
Transparent, elastic silicon sheet (thickness: 0.0254 cm) (Specialty manufacturing INC, USA) was punched out into round sheets (diameter: 1.4 cm). Glass slips (diameter: 1.4 cm) (Polylabo, France) and silicon sheets were sterilised by overnight immersion in 75% ethanol aqueous solution, air- dried, then placed in a 24-well multiwell culture plate, and sterilised by 10 min UV irradiation. Gelatin solutions (1% w/v in distilled water) (Sigma, France) were sterilised by filtration before use. Cells were seeded into 24 wells culture plates at a density of 3 × 104 cells/cm2 on different surface glass slips (control surface), silicon sheet, coated or not with gelatin for 24 h at 37°C to allow cell attachment.
Biochemical Stimuli
The concentrations of growth factors used in this study included those noted to be effective in previous studies.37,42 The concentration range for TGF-β was 0.12–25 ng ml−1 and from 1 to 100 ng ml−1 for PDGF-AB. In our study, five concentrations of TGF-β1 (0.1, 0.5, 1, 5, 10 ng ml−1) and four concentrations for PDGF-AB (1, 5, 10 and 50 ng ml−1) were used. These concentrations were added to the cultures in order to determine their effects on the cell proliferation for PDGF-AB and collagen production for TGF-β1. Cell proliferation and collagen synthesis were determined 72 h after the addition of the growth factors. Culture medium containing 10%FBS without growth factors was used as control.
Fibroblast Proliferation Experiments
MTT Assay
After 72 h PDGF-AB treatment, MTT 3-[4,5-Dimethylthiazol-2,5-diphenyltetrazolium bromide, blue Thiazolyl] (Sigma, France) was prepared in PBS at a final concentration of 2 mg/ml. Then 125 μl of the solution were added to each well containing 500 μl of DMEM without red phenol. The cells were incubated for 4 h at 37°C to allow the yellow dye to be transformed into blue formazan crystals by the mitochondrial dehydrogenases. The insoluble product was dissolved by addition of 800 μl of acidified isopropanol (0.04 N HCl in isopropanol), and thoroughly mixed to dissolve the dark blue crystals. The supernatants were removed, centrifuged, and read within 30 min by using a Beckman DU-600 spectrophotometer (USA) with a wavelength of 570 and 700 nm was used as reference wavelength. The optical density difference OD = OD570 nm−OD700 nm was estimated.
Cell Cycle Analysis
After 72 h PDGF-AB treatment, cells were trypsinisation then collected by centrifugation (500g) for 10 min. Cells pellets were washed twice with 2 ml of PBS. 50 μl of the washed cell suspension (15 × 104 cells) were stained according the kit instruction (Coulter DNA Prep Reagents kit; Beckman-Coulter, Miami, USA) in order to analyse the cell cycle by flow cytometry (EPICS XL Coulter; Beckman-Coulter). Primary histograms were generated by gating the cells according to the surface and the peak of the fluorescence signal in order to avoid taking doublets into account. These histograms were then submitted to computerised calculations of the cell cycle by using polynomial analysis derived from the Dean and Jett model Multi Cycle AV (Phoenix Flow System, San Diego, USA). For each testing condition, the percentage of cells in each phase was determined. DNA content was analysed in at least 105 nuclei in each sample.
RNA Isolation and RT-PCR
Total cellular RNA were isolated from fibroblasts after dissolution in TRIzol reagent (Invitrogen, France) according to the manufacturer’s instructions. RNA were extracted using chloroform, then samples were centrifuged (9500g) for 15 min at 4°C. Isopropanol was added to precipitate the RNA and the samples were centrifuged for 10 min under the same conditions. The RNA pellet was washed by addition of 1 ml of 75% ethanol and centrifuged at 5600g for 5 min. The pellet was resuspended in 50 μl DEPC-treated water. The concentrations of RNA were determined spectrophotometrically at 260 nm and the purity of RNA were controlled by the ratio DO260 nm/DO280 nm. The first strand cDNA was prepared from the RNA with reverse transcriptase (kit ThermoScript RT-PCR System) (BioRad, USA). The cDNA was amplified by polymerase chain reaction (Platinum Taq DNA Polymerase) (Invitrogen, France) using the primers shown in Table 1. Gel electrophoresis of RT-PCR products was performed in a 2% agarose (Sigma, France) run in 0.5× Tris/-boric acid EDTA (TBE) buffer (Bio-Rad, USA). The gel was stained in a 0.5 μg/ml ethidium bromide solution for 20 min and the images were obtained using a Bio-Rad GS-2000 Molecular Imaging System. Bands intensities were evaluated against background quantified by Quantity One Image software (Bio-Rad, USA) and normalized to β-actin.
TABLE 1.
Prime sequence for RT-PCR.
| MRNA | Primer sequence | Product size (bp) |
|---|---|---|
| Type I collagen | 5′-TCC CCA GCC ACA AAG AGT CTA CA-3′ | 155 |
| 5′-GTG ATT GGT GGG ATG TCT TCG TC-3′ | ||
| Type III collagen | 5′-CTG CCA TCC TGA ACT CAA GAG TGG-3′ | 447 |
| 5′-CCA TCC TCC AGA ACT GTG TAG G-3′ | ||
| Β-actin | 5′-ATC TGG CAC CAC ACC TTC TAC AAT GAG CTG CG-3′ | 838 |
| 5′-CGT CAT ACT CCT GCT TGC TGA TCC ACA TCT GC-3′ |
Immuonofluorescence Labelling
After incubation with TGF-β1, the cells were rinsed with PBS. The cells were incubated for 45 min with anti-collagen I (1/50 dilution) or anti-collagen III (1/100 dilution) rabbit IgG polyclonal (Calbiochem, France) in DMEM without red phenol (GibcoBRL, Life Technologie, France) containing 0.5% (w/v) BSA. The cells were then rinsed thoroughly in DMEM without red phenol and incubated with the second antibody, Alexa™-488 conjugated goat anti-rabbit IgG (λex = 488 nm, λem = 515 nm) (1/200 dilution) for 30 min in the dark. After washing with DMEM without red phenol, the cells were fixed with 1% para-formaldehyde (Sigma, France) in PBS for 10 min, and rinsed in PBS. The cells were observed under a fluorescence confocal microscope (SP2-Leica micro systems, Germany).
Statistical Analysis
Differences in cell proliferation and collagen synthesis were analysed by one-way ANOVA analysis to evaluate differences between groups with PLSD Fisher correction (Statview IV®, Abacus Concepts Inc, Berkley,CA, USA). A p value less than 0.05 was considered statistically significant. All data were expressed as the mean ± standard deviation (SD).
RESULTS
All the works were realized on three types of substrates (silicon sheet, silicon sheet with 1% gelatin coating as testing surfaces and glass surface used as a control).
Effect of PDGF-AB on Cell Proliferation
We studied the effect of PDGF-AB on fibroblast proliferation according to substrates and PDGF concentrations by two different and complementary methods.
MTT Assays
The results of the MTT assay are summarized in Fig. 1. These results were obtained from fibroblast cultured on different substrates at day 3 in the presence or absence of four different concentrations of PDGF-AB. On the glass and gelatin coated silicon sheet cultures, PDGF-AB induced a dose-dependent increase in the value of A570. On glass culture, the significant increase was observed in the range of 5–50 ng ml−1 PDGF-AB (p < 0.01). On the gelatin coated silicon sheet culture, the significant increase was observed in the range of 1–50 ng ml−1 PDGF-AB (p < 0.01). On the silicon sheet culture, the values of A570 decrease when the concentration of PDGF-AB was above 5 ng ml−1.
FIGURE 1.
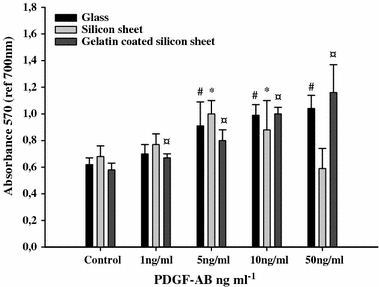
Dose dependent effect of PDGF-AB on cell proliferation by MTT assays. Cells were treated with different concentrations of PDGF-AB for 72 h. The metabolic activity of fibroblast seeded on glass, silicon sheet with or without 1% gelatin was measured by MTT assays. # p < 0.05 versus control glass, *p < 0.05 versus control silicon sheet, ¤ p < 0.05 versus control gelatin coated silicon sheet, n = 12.
Nevertheless, we observed a loss of cells on silicon sheet at 50 ng ml−1 PDGF-AB.
Cell Cycle Analysis
To confirm the MTT results, we also studied the effect of PDGF-AB on cell cycle (Figs. 2 and 3). Cell cycle analysis was performed on day 3 of the culture. It was observed in the three types of substrates that PDGF-AB induced a concentration-dependant decrease in the percentage of cells in G0/G1 phase and an increase in S and G2/M phase.
FIGURE 2.
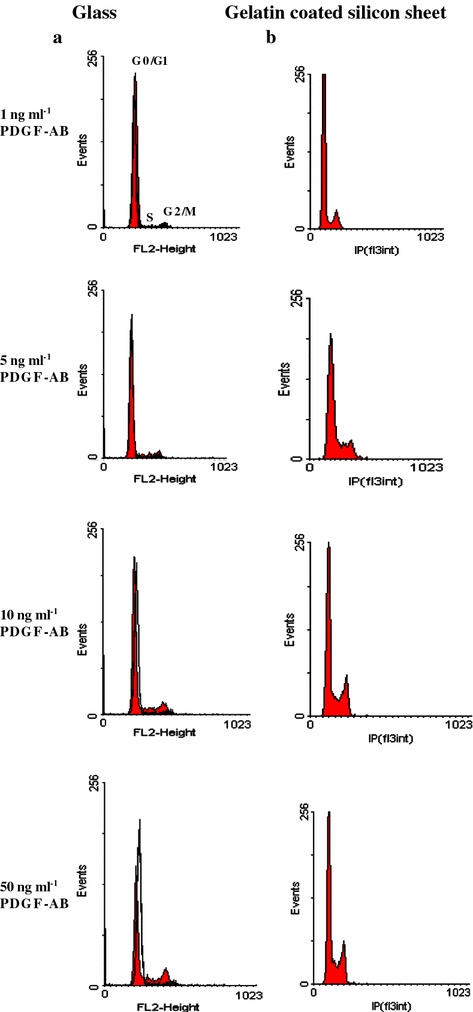
Cell cycle analysis by flow cytometry. Representative cell cycle histogram of the cells cultured on glass (a), gelatin coated silicon sheet (b). After 3 days of treatment with PDGF-AB (1, 5, 10, 50 ng ml−1), cells were recovered and DNA staining with propidium iodide (IP) was performed. Ten thousand cells were analysed for each sample, n = 9.
FIGURE 3.
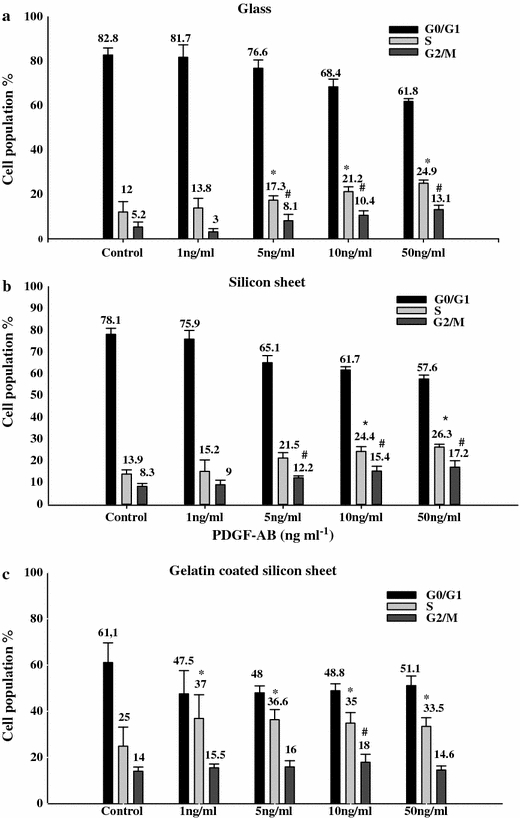
Effect of different concentrations of PDGF-AB on fibroblast cell cycle regulation. Cells cultured on glass (a), silicon sheet (b), gelatin coated silicon sheet (c). Cells were subjected to different concentrations of PDGF-AB (1, 5, 10, 50 ng ml−1). The percentage of cells in each phase of the cell cycle was analysed by flow cytometry. Results are represented as mean ± SD of three independent experiments for each substrate. p < 0.05 versus control, n = 9.
On gelatin coated silicon sheet, the percentage of cells in S-phase was higher than that without gelatin or glass (Fig. 3C). Moreover, cells exposed to 10 ng ml−1 of PDGF-AB had the highest percentage of cells in G2/M phase (p < 0.01).
Effect of TGF-β1 on the Expression of Collagens
Effect of TGF-β1 on Type I and Type III Collagens mRNA Expressions
The gene expressions of collagens in cells cultured on different substrates were measured by RT-PCR. RT-PCR analysis of type I and type III collagen were performed at day 3 in the presence or absence of five different concentrations of TGF-β1. The RT-PCR results on human fibroblasts seeded on the three substrates are shown in Fig. 4. The quantitative results concerning type I and type III collagen are summarised in Fig. 5.
FIGURE 4.
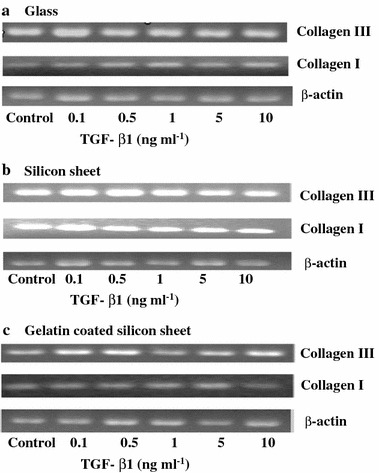
Effect of different concentrations of TGF-β1 on the mRNA expression of type I and type III collagen. Representative RT-PCR results of human fibroblasts seeded on glass (a), silicon sheet (b), gelatin coated silicon sheet (c). The OD of each band was normalised to the values of housekeeping gene, β-actin. The cells were cultured on different substrates in the presence of different concentrations of TGF-β1 (0.1, 0.5, 1, 5, 10 ng ml−1) for 3 days.
FIGURE 5.
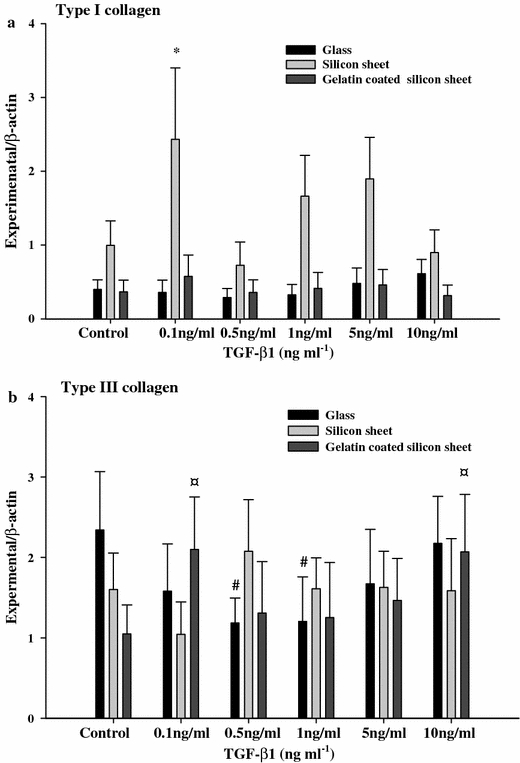
Quantitative measurement of the RT-PCR results. a: type I collagen, b: type III collagen. Results are expressed as mean ± SD of a representative of three different experiments carried out in duplicate. Black bars represent cell culture on glass; bright grey bars represent cell culture on silicon sheet; dark grey bars represent cell culture on gelatin coated silicon sheet. # p < 0.05 versus control glass, *p < 0.05 versus control silicon sheet, ¤ p < 0.05 versus control gelatin coated silicon sheet, n = 6.
The mRNA expression of collagen type I is shown in Fig. 5a. On the glass culture and gelatin coated silicon sheet cultures, there was no significant difference in the mRNA expression of collagen type I between the TGF-β1 treated group and the control group. Otherwise, on the silicon sheet culture, the mRNA expression of collagen type I increased significantly in the presence of 0.1 ng ml−1 TGF-β1.
The mRNA expression of collagen type III is shown in Fig. 5b. On the glass culture, we can see a cyclic effect of TGF-β1 on the expression of collagen type III and it reached a maximum response at 10 ng ml−1 TGF-β. Also, compared to control group, the mRNA expression of collagen type III was decreased significantly at a concentration of 0.5 and 1 ng ml−1 TGF-β1 (p < 0.05).
On the gelatin coated silicon sheet cultures, the significant increase in the mRNA expression of collagen type III was observed by the addition of 0.1 and 10 ng ml−1 TGF-β1 (p < 0.05).
On the silicon sheet culture, the mRNA expression of collagen type III decrease when the concentration of TGF-β1was above 0.5 ng ml−1.
Qualitative Expression of Type I and Type III Collagens
In parallel of the RT-PCR experiments, we carried out some experiments by confocal microscopy to visualise the expression of collagen. The protein expression of collagen type I and type III of the cells cultured on glass were revealed by indirect immunofluorescence staining. As shown in Fig. 6, collagen type I occurred as spots dispersed around the cells, and collagen type III was organised as network-like fibre. There is no difference in the expression of type I collagen with the different concentration of TGF-β1. In contrast, the expression of type III collagen was increased after the addition of TGF-β1. The same expression model of collagens was observed on the other substrates (data not shown).
FIGURE 6.
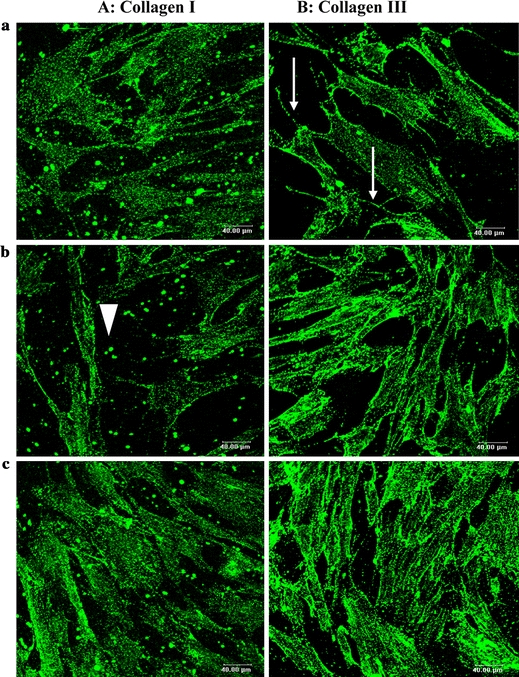
Immunofluorescence staining of collagen in monolayer cultures of fibroblast on glass visualised by confocal microscopy. (A) Type I collagen is identified on the 3rd day of culture by use of anti collagen type I antibodies. Only spots of this type of collagen were secreted by the cells (arrowhead). (B) Type III collagen is identified by use of anti collagen type III antibodies. It appears like network in the cell (arrow). (objectif 40×/NA 0.80, Leica, Germany). Bar 40 μm. (a): Control cell (DMEM + 10%FBS); (b): Cell treated with 0.5 ng ml−1 TGF-β1; (c): Cell treated with 10 ng ml−1 TGF-β1.
DISCUSSION
As cell proliferation and matrix synthesis are important parameters in tissue engineering, the aim of this study was to optimise the in vitro culture conditions on ligament/tendon tissues for future experiments in bioreactors. The effect of PDGF-AB and TGF-β1 on the cell proliferation and matrix synthesis of fibroblast cultured on three different types of substrates (silicon sheet with or without 1% gelatin, glass) for 3 days were investigated in this study. For the tissue regeneration, it is better to use three-dimensional scaffolds to investigate the effects of the properties of the material on cell behaviour. However, there are many parameters of the scaffolds to be considered, such as porosity, pore size, pore interconnectivity, surface property, topography and chemical composition. Therefore, in this study, two-dimensional substrates were used to investigate the influence of substrate surface property on the proliferation and ECM synthesis of fibroblast.
Firstly, we used PDGF-AB, which was widely used as a mitogenic growth factor and as a chemotactic agent for cells such as fibroblasts.45 Our experiments demonstrated that cell proliferation was enhanced by PDGF-AB. This result was consistent with those of the previous studies on lung fibroblast,6,30 canine anterior cruciate ligament cell,8 human periodontal ligament (PDL) cells36 and human anterior cruciate ligament cell (ACL).33 PDGF increased the number of fibroblasts available to enter the cell replication cycle by stimulating termination of the G0 phase of the cell cycle. The results obtained in this study are similar to those reported by Letson27 and Schmidt46 which showed that PDGF would be an effective growth factor that stimulates resting cells to advance from G0 to G1 of the cell cycle.
Although the addition of PDGF-AB resulted in significant increase in cell proliferation on all the substrates, the highest increase was found on gelatin coated silicon sheet culture. This result may be explained by the physical properties of the substrate surface. High cell proliferation was observed for the substrates with contact angles of 60–80° as well as on protein coated culture plates such as gelatin.18 Park et al. have demonstrated that mescenchymal stem cells (MSCs) cultured on collagen I or gelatin coated membranes expressed similar levels of smooth muscles (SM) markers.41 Hori et al. reported that the morphology and the proliferation of MSCs were influenced by the type of substrate and culture medium.18 The use of gelatin coated silicon sheet as a substrate allows to enhance the cell adhesion, the differentiation and the proliferation.
In addition, the higher response was found to 10 ng ml−1 PDGF on gelatin coated silicon sheet culture but not to the maximal dose as 50 ng ml−1 PDGF-AB. This finding indicates that it exist a dose-dependant effect of PDGF-AB on cell proliferation in this culture model and 10 ng ml−1 PDGF might be the optimal concentration. Indeed, our result was consisting with the previous experiment which demonstrated that 10 ng ml−1 of PDGF promoted fibroblast attachment to collagen coated plates and proliferation.21,36
Secondly, we investigated the effect of TGF-β1 on collagen synthesis. TGF-β1 is the prototype of a large family of growth factors regulating cell growth, development, differentiation, immune system and tissue repair. On the one hand, TGF-β1 mostly inhibited the cell growth both in vivo and in vitro.2 It could arrests cell cycle progression at a point in late G1 phase.12,19,43 Furthermore, stimulatory effects of PDGF-AB have been demonstrated to be blocked by the presence of TGF-β1.20,23 On the other hand, it has been shown that TGF-β regulates production of the ECM, including collagen, fibronectin and tenascin.11,16,40,44
Therefore, in the second step of our works, we study the effect of TGF-β1 on the ECM synthesis. Our data showed that the exogenous TGF-β1 influenced the expression of type I and type III collagen in a substrate- dependant manner.
In our study, the mRNA expression of collagen type III was higher than that of collagen type I when the cells were cultured on glass and gelatin coated silicon sheet for 72 h. During the early phases of ligament healing and remodelling more type III collagen is produced than type I collagen.1,10,49 Therefore the greater upregulation of type III collagen as compared to type I collagen is a positive indicator that the cells are secreting signals for the healing and remodelling process.
However, on glass, we observed the highest expression of type III collagen mRNA in the control group. In the present work, we used culture medium containing 10% FBS. Although, we did not add any TGF-β1 in the medium, it seemed that this increase was due to the presence of growth factors in the serum and which normally stimulate protein synthesis and other different biological processes.
Furthermore, we found that TGF-β1 effected the expression of type I and type III collagen synthesis by fibroblasts with a dose dependant manner. The expression of type III collagen mRNA on gelatin coated silicon sheet was significantly increased at 0.1 and 10 ng ml−1 TGF-β1. The results of this study are consistent with the previous work which demonstrated that 10 ng ml−1 of TGF-β1 may promote fibroblast attachment.21 In 2D cell culture, TGF-β1 has been demonstrated to have a dose-dependent effect on collagen production.4,7,28,29,32,34
In summary, our results demonstrated that PDGF-AB promoted the cell proliferation, which is a basic step in the process of ligament repair. Our data also suggest that TGF-β1 is an important cytokine during the early phases of ligament healing process for its role in the promotion of extra cellular matrix formation. These results allowed us to determine the main biochemical conditions of in vitro construction of ligament/tendon tissues.
Our results implied that it is better to use protein-coating substrate to improve the proliferation and the synthesis of collagen for later mechanical stretching study. Further studies are needed to determine the combination of these two growth factors and their effect on the biological processes.
ACKNOWLEDGMENTS
This work was partly supported by the fellowship from French CNRS/SPI (program ATIP). We also thank the Foundation of Medical Research for supporting this work.
REFERENCES
- 1.Amiel D., Kleiner J. B., Roux R. D., Harwood F. L., Akeson W. H. (1986) The phenomenon of “ligamentization”: Anterior cruciate ligament reconstruction with autogenous patellar tendon J. Orthop. Res. 4: 162–172 [DOI] [PubMed]
- 2.Amiel D., Nagineni C. N., Choi S. H., Lee J. (1995) Intrinsic properties of ACL and MCL cells and their responses to growth factors Med. Sci. Sports Exerc. 27: 844–851 [PubMed]
- 3.Aspenberg P., Forslund C. (1999) Enhanced tendon healing with GDF 5 and 6 Acta Orthop. Scand. 70:51–54 [DOI] [PubMed]
- 4.Bosman F. T., Stamenkovic I. (2003) Functional structure and composition of the extracellular matrix J. Pathol. 200:423–428 [DOI] [PubMed]
- 5.Boura C., Menu P., Payan E., Picart C., Voegel J. C., Muller S., Stoltz J. F. (2003) Endothelial cells grown on thin polyelectrolyte mutlilayered films: An evaluation of a new versatile surface modification Biomaterials 24:3521–3530 [DOI] [PubMed]
- 6.Clark J. G., Madtes D. K., Raghu G. (1993) Effects of platelet-derived growth factor isoforms on human lung fibroblast proliferation and procollagen gene expression Exp. Lung Res. 19:327–344 [DOI] [PubMed]
- 7.Deie M., Marui T., Allen C. R., Hildebrand K. A., Georgescu H. I., Niyibizi C., Woo S. L. (1997) The effect of age on rabbit MCL fibroblast matrix synthesis in response to TGF-β1 or EGF Mech. Aging Dev. 97:121–130 [DOI] [PubMed]
- 8.DesRosiers E. A., Yahia L., Rivard C. H. (1996) Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors J. Orthop. Res. 14:200–208 [DOI] [PubMed]
- 9.Dunn M. G., Liesch J. B., Tiku M. L., Zawadsky J. P. (1995) Development of fibroblast-seeded ligament analogs for ACL reconstruction J. Biomed. Mater. Res. 29:1363–1371 [DOI] [PubMed]
- 10.Eckes B., Zigrino P., Kessler D., Holtkotter O., Shephard P., Mauch C., Krieg T. (2000) Fibroblast matrix interactions in wound healing and fibrosis Matrix Biol. 19:325–332 [DOI] [PubMed]
- 11.Eickelberg O., Kohler E., Reichenberger F., Bertschin S., Woodtli T., Erne P., Perruchoud A. P., Roth M. (1999) Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3 Am. J. Physiol. 276:L814–L824 [DOI] [PubMed]
- 12.Shalaw F. G., Slimani S., Kolopp-Sarda M. N., Marchand M., Faure G., Stoltz J. F., Muller S. (2006) Effect of cyclic stretching and foetal bovine serum (FBS) on proliferation and extra cellular matrix synthesis of fibroblast. Biomed. Mater. Eng. 16:S137–S144 [PubMed]
- 13.Ge Z., Yang F., Goh J. C., Ramakrishna S., Lee E. H. (2006) Biomaterials and scaffolds for ligament tissue engineering J. Biomed. Mater. Res. A 77:639–652 [DOI] [PubMed]
- 14.Goh J. C., Ouyang H. W., Teoh S. H., Chan C. K., Lee E. H. (2003) Tissue-engineering approach to the repair and regeneration of tendons and ligaments Tissue Eng. 9:S31–S44 [DOI] [PubMed]
- 15.Health C. A. (2005) Cells for tissue engineering Trends Biotechnol. 18:17–19 [DOI] [PubMed]
- 16.Hetzel M., Bachem M., Anders D., Trischler G., Faehling M. (2005) Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts Lung 183:225–237 [DOI] [PubMed]
- 17.Hildebrand K. A., Woo S. L., Smith D. W., Allen C. R., Deie M., Taylor B. J., Schmidt C. C. (1998) The effect of patelet -drived growth factor-BB on healing of the rabbit medial collateral ligament Am. J. Sports Med. 26:549–554 [DOI] [PubMed]
- 18.Hori Y., Inoue S., Hirano Y., Tabata Y. (2004) Effect of culture substrates and fibroblast growth factor addition on the proliferation and differentiation of rat bone marrow stromal cells Tissue Eng. 10:995–1005 [DOI] [PubMed]
- 19.Howe P. H., Draetta G., Leof E. B. (1991) Transforming growth factor beta 1 inhibition of p34cdc2 phosphorylation and histone H1 kinase activity is associated with G1/S-phase growth arrest Mol. Cell Biol. 11:1185–1194 [DOI] [PMC free article] [PubMed]
- 20.Hung L. M., Tsai C. H., Chen J. K. (1997) TGF-beta1 selectively suppresses PDGF receptor signaling pathways in MG-63 human osteosarcoma cell Life Sci. 61:685–693 [DOI] [PubMed]
- 21.Ihn H., Kikuchi K., Soma Y., Sato S., Fujimoto M., Tamaki T., Igarashi A., Takehara K. (1995) The stimulatory effects of PDGF and TGF-beta 1 on dermal fibroblast attachment Acta Derm. Venereol. 75:367–371 [DOI] [PubMed]
- 22.Kim S. G., Akaike T., Sasagaw T., Atomi Y., Kurosawa H. (2002) Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells Cell Struct. Funct. 27:139–144 [DOI] [PubMed]
- 23.Kletsas D., Stathakos D., Sorrentino V., Philipson L. (1995) The growth-inhibitory block of TGF-beta is located close to the G1/S border in the cell cycle Exp. Cell Res. 217:477–483 [DOI] [PubMed]
- 24.Kuroda R., Kurosaka M., Yoshiya S., Mizuno K. (2000) Localization of growth factors in the reconstructed anterior cruciate ligament: Immunohistological study in dogs Knee Surg. Sports Traumatol. Arthrosc. 8:120–126 [DOI] [PubMed]
- 25.Langer R., Vacanti J. P. (1993) Tissue engineering Science 260:920–926 [DOI] [PubMed]
- 26.Lee J., Harwood F. L., Akeson W. H., Amiel D. (1998) Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments Iowa Orthop. J. 18:19–25 [PMC free article] [PubMed]
- 27.Letson A. K., Dahners L. E. (1994) The effect of combinations of growth factors on ligament healing Clin. Orthop. 308:207–212 [PubMed]
- 28.Lieb E., Vogel T., Milz S., Dauner M., Schulz M. B. (2004) Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. I. Culture conditions and tissue formation Tissue Eng. 10:1399–1413 [DOI] [PubMed]
- 29.Lieb E., Vogel T., Milz S., Dauner M., Schulz M. B. (2004) Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation Tissue Eng. 10:1414–1425 [DOI] [PubMed]
- 30.Liebeskind A., Srinivasan S., Kaetzel D., Bruce M. (2000) Retinoic acid stimulates immature lung fibroblast growth via a PDGF-mediated autocrine mechanism Am. J. Physiol. Lung Cell Mol. Physiol. 279:L81–L90 [DOI] [PubMed]
- 31.Liu S. H., Yang R. S., al-Shaikh R., Lane J. M. (1995) Collagen in tendon, ligament, and bone healing. A current review Clin. Orthop. 318:265–278 [PubMed]
- 32.Marui T., Niyibizi C., Georgescu H. I., Cao M., Kavalkovich K. W., Levine R. E., Woo S. L. (1997) Effect of growth factors on matrix synthesis by ligament fibroblasts J. Orthop. Res. 15:18–23 [DOI] [PubMed]
- 33.Meaney M. M., Rice K., Wright R. J., Spector M. (2003) The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold J. Orthop. Res. 21:238–244 [DOI] [PubMed]
- 34.Murphy P. G., Loitz B. J., Frank C. B., Hart D. A. (1994) Influence of exogenous growth factors on the synthesis and secretion of collagen types I and III by explants of normal and healing rabbit ligaments Biochem. Cell Biol. 72:403–409 [DOI] [PubMed]
- 35.Nakatani T., Marui T., Hitora T., Doita M., Nishida K., Kurosaka M. (2002) Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1 J. Orthop. Res. 20:1380–1386 [DOI] [PubMed]
- 36.Ojima Y., Mizuno M., Kuboki Y., Komori T. (2003) In vitro effect of platelet-derived growth factor-BB on collagen synthesis and proliferation of human periodontal ligament cells Oral Dis. 9:144–151 [DOI] [PubMed]
- 37.Orr F. W., Millar-Book W., Singh G. (1990) Chemotactic activity of bone and platelet-derived TGF-beta for bone-metastasizing rat Walker 256 carcinosarcoma cells Invasion Metastasis. 0:241–252 [PubMed]
- 38.Ouyang, H. W., J. C. H. Goh, X. M. Mo, S. H. Teoh, S. W. Chong, E. H. Lee. Study on cell–material systems for ACL regeneration: Ligament cells and mesenchymal stem cells on various biodegradable polymeric films. Mater. Sci. Eng. C Bio. S20:63, 2002
- 39.Ouyang H. W., Goh J. C. H., Mo X. M., Teoh S. H., Lee E. H. (2002) Characterization of anterior cruciate ligament cells and bone marrow stromal cells on various biodegradable polymeric films Mater. Sci. Eng. C 20:63–69 [DOI]
- 40.Papakonstantinou E., Roth M., Tamm M., Eickelberg O., Perruchoud A. P., Karakiulakis G. (2002) Hypoxia differentially enhances the effects of transforming growth factor-beta isoforms on the synthesis and secretion of glycosaminoglycans by human lung fibroblasts J. Pharmacol. Exp. Ther. 301:830–837 [DOI] [PubMed]
- 41.Park J. S., Chu J. S., Cheng C., Chen F., Chen D., Li S. (2004) Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells Biotechnol. Bioeng. 88:359–368 [DOI] [PubMed]
- 42.Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. (1989) Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J. Cell Biol. 109:429–440 [DOI] [PMC free article] [PubMed]
- 43.Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Munger K., Howley P. M., Moses H. L. (1990) TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains Cell 61:777–785 [DOI] [PubMed]
- 44.Roberts A. B. (1998) Molecular and cell biology of TGF-beta Miner Electrol. Metab. 24:111–119 [DOI] [PubMed]
- 45.Ross R., Raines E. W., Bowen-Pope D. F. (1986) The biology of platelet-derived growth factor Cell 46:155–169 [DOI] [PubMed]
- 46.Schmidt C. C., Georgescu H. I., Kwoh C. K., Blomstrom G. L., Engle C. P., Larkin L. A., Evans C. H., Woo S. L. (1995) Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments J. Orthop. Res. 13:184–190 [DOI] [PubMed]
- 47.Spindler K. P., Imor A. K., Mayes C. E., Davidson J. M. (1996) Patellar tendon and anterior cruciate ligament have different mitogenic responses to platelet-derived growth factor and transforming growth factor β J. Orthop. Res. 14:542–546 [DOI] [PubMed]
- 48.Tabata Y. (2001) Recent progress in tissue engineering Drug Discov. Today 6:483–487 [DOI] [PubMed]
- 49.Woo S. L., Hildebrand K., Watanabe N., Fenwick J. A., Papageorgiou C. D., Wang J. H. (1999) Tissue engineering of ligament and tendon healing Clin. Orthop. 367:S312–S323 [DOI] [PubMed]


