Abstract
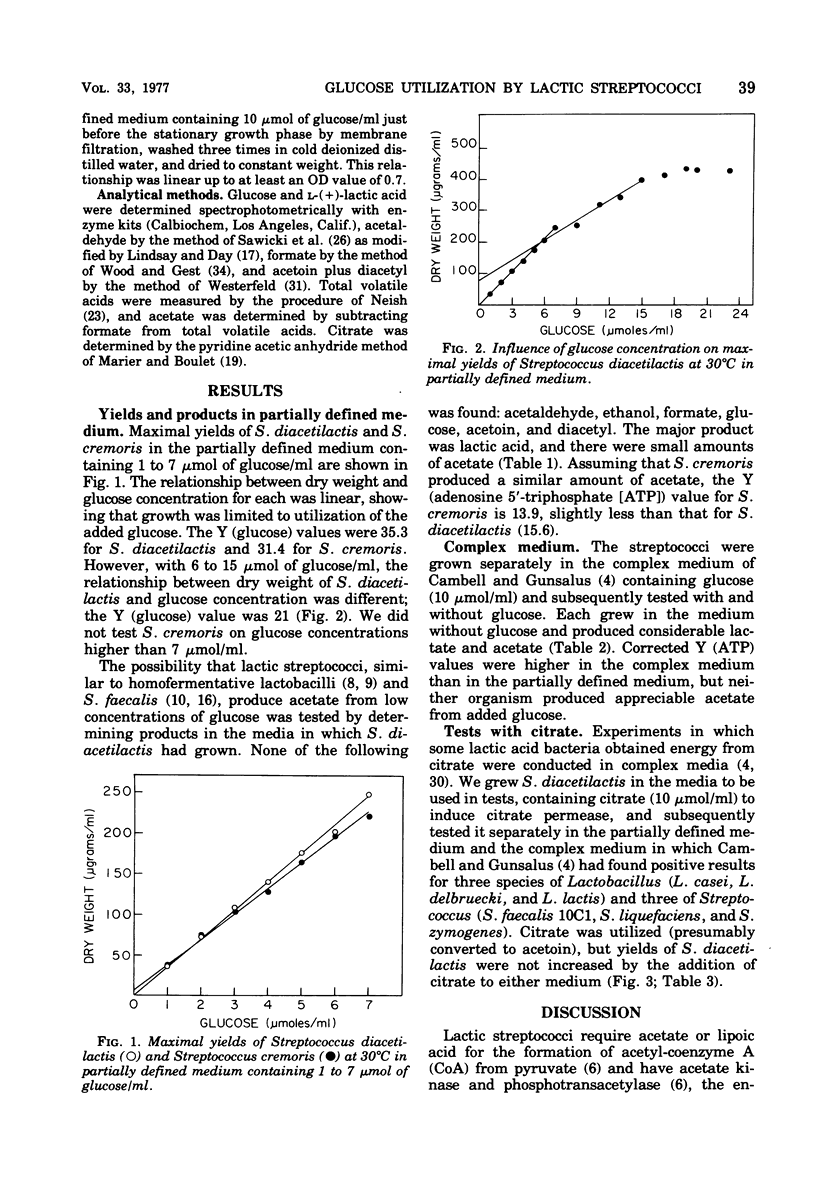
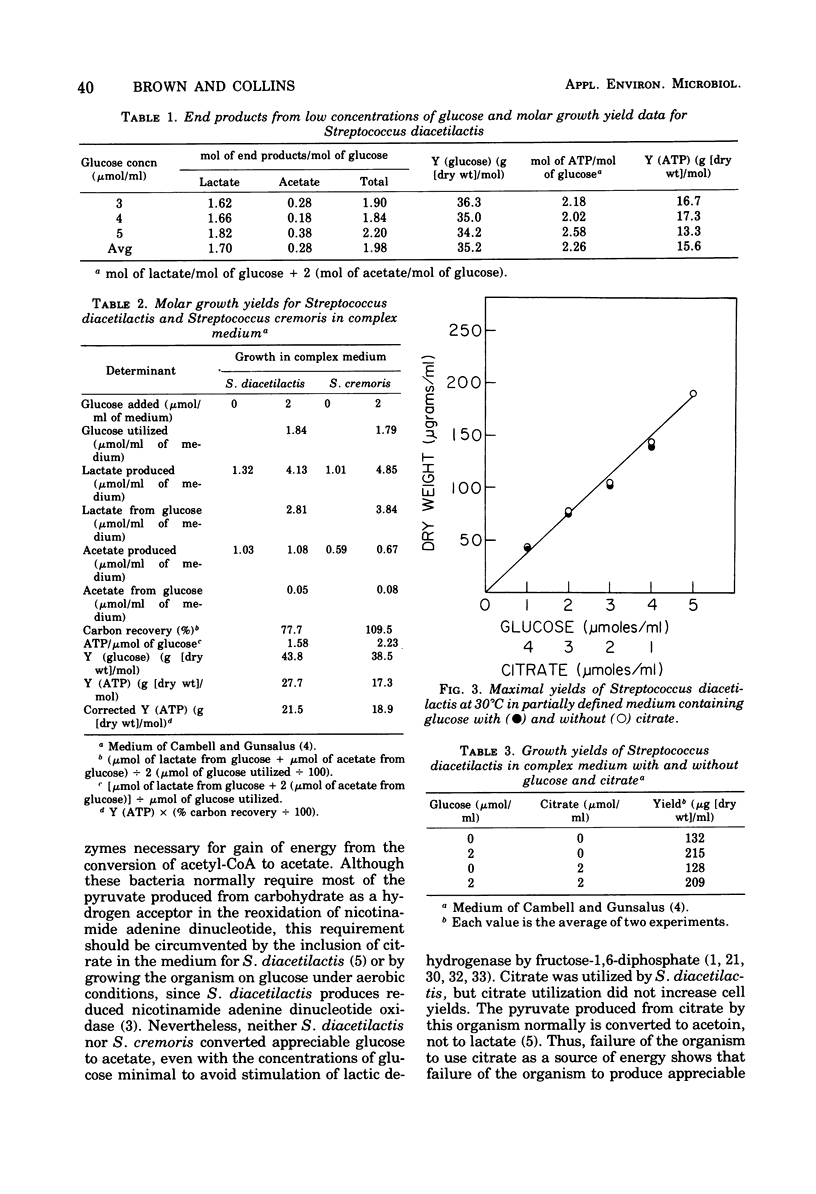
Maximum acetate produced aerobically by Streptococcus diacetilactis and Streptococcus cremoris was 14% of 1 to 7 mumol of glucose/ml in a partially defined medium that contained lipoic acid. Y (glucose) values were 35.3 (S. diacetilactis) and 31.4 (S. cremoris) with low concentrations (1 to 7 mumol/ml) of glucose in the medium and 21 (S. diacetilactis) with higher concentrations (6 to 15 mumol/ml). Y (adenosine 5'-triphosphate) values for the bacteria, determined by taking into account the end products produced, were 15.6 and 13.9 for S. diacetilactis and S. cremoris, respectively, in the partially defined medium containing 1 to 7 mumol of glucose/ml and higher (21.5 and 18.9, respectively) in a complex medium that contained 2 mumol of glucose/ml. Addition of citrate in addition to glucose did not result in higher molar growth yields.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn J. C., Collins E. B. Reduced nicotinamide adenine dinucleotide oxidase of Streptococcus diacetilactis. J Dairy Sci. 1970 Jul;53(7):857–860. doi: 10.3168/jds.S0022-0302(70)86307-X. [DOI] [PubMed] [Google Scholar]
- Campbell J. J., Gunsalus I. C. Citric Acid Fermentation by Streptococci and Lactobacilli. J Bacteriol. 1944 Jul;48(1):71–76. doi: 10.1128/jb.48.1.71-76.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins E. B., Bruhn J. C. Roles of acetate and pyruvate in the metabolism of Streptococcus diacetilactis. J Bacteriol. 1970 Sep;103(3):541–546. doi: 10.1128/jb.103.3.541-546.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirar H., Collins E. B. Aerobic utilization of low concentrations of galactose by Lactobacillus plantarum. J Gen Microbiol. 1973 Oct;78(2):211–215. doi: 10.1099/00221287-78-2-211. [DOI] [PubMed] [Google Scholar]
- Dirar H., Collins E. B. End-products, fermentation balances and molar growth yields of homofermentative lactobacilli. J Gen Microbiol. 1972 Nov;73(2):233–238. doi: 10.1099/00221287-73-2-233. [DOI] [PubMed] [Google Scholar]
- FORREST W. W., WALKER D. J. SYNTHESIS OF RESERVE MATERIALS FOR ENDOGENOUS METABOLISM IN STREPTOCOCCUS FAECALIS. J Bacteriol. 1965 Jun;89:1448–1452. doi: 10.1128/jb.89.6.1448-1452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNSALUS I. C., DOLIN M. I., STRUGLIA L. Pyruvic acid metabolism. III. A manometric assay for pyruvate oxidation factor. J Biol Chem. 1952 Feb;194(2):849–857. [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON P. N. CONTINUOUS CULTURE OF SOME ANEROBIC AND FACULTATIVELY ANAEROBIC RUMEN BACTERIA. J Gen Microbiol. 1965 Feb;38:167–180. doi: 10.1099/00221287-38-2-167. [DOI] [PubMed] [Google Scholar]
- Hobson P. N., Summers R. The continuous culture of anaerobic bacteria. J Gen Microbiol. 1967 Apr;47(1):53–65. doi: 10.1099/00221287-47-1-53. [DOI] [PubMed] [Google Scholar]
- Johnson M. G., Collins E. B. Synthesis of lipoic acid by Streptococcus faecalis 10C1 and end-products produced anaerobically from low concentrations of glucose. J Gen Microbiol. 1973 Sep;78(1):47–55. doi: 10.1099/00221287-78-1-47. [DOI] [PubMed] [Google Scholar]
- LINDSAY R. C., DAY E. A. RAPID QUANTITATIVE METHOD FOR DETERMINATION OF ACETALDEHYDE IN LACTIC STARTER CULTURES. J Dairy Sci. 1965 Jun;48:665–669. doi: 10.3168/jds.s0022-0302(65)88318-7. [DOI] [PubMed] [Google Scholar]
- Maeng W. J., Baldwin R. L. Factors influencing rumen microbial growth rates and yields: effects of urea and amino acids over time. J Dairy Sci. 1976 Apr;59(4):643–647. doi: 10.3168/jds.S0022-0302(76)84253-1. [DOI] [PubMed] [Google Scholar]
- Mou L., Mulvena D. P., Jonas H. A., Jago G. R. Purification and properties of nicotinamide adenine dinucleotide-dependent D- and L- lactate dehydrogenases in a group N streptococcus. J Bacteriol. 1972 Aug;111(2):392–396. doi: 10.1128/jb.111.2.392-396.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa H. H., Collins E. B. Molar growth yields of certain lactic acid bacteria as influenced by autolysis. J Bacteriol. 1968 Jul;96(1):117–125. doi: 10.1128/jb.96.1.117-125.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'KANE D. J. Influence of the pyruvate oxidation factor on the oxidative metabolism of glucose by Streptococcus faecalis. J Bacteriol. 1950 Oct;60(4):449–458. doi: 10.1128/jb.60.4.449-458.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'kane D. J., Gunsalus I. C. Pyruvic Acid Metabolism: A Factor Required for Oxidation by Streptococcus faecalis. J Bacteriol. 1948 Oct;56(4):499–506. doi: 10.1128/jb.56.4.499-506.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]


