Introduction
Cytomegalovirus retinitis (CMVR) is a major opportunistic complication of the acquired immune deficiency syndrome (AIDS). In the developed world, prior to the availability of highly active anti-retroviral therapy (HAART), it was estimated that about 30% of patients with AIDS would develop CMVR during their lifetime. However, since the introduction of HAART, the incidence of CMVR has declined significantly in these countries. By far the most valuable intervention in the treatment of CMVR is the treatment of the underlying HIV disease with HAART. HAART is unfortunately not widely available in the developing world and it is here that the AIDS epidemic is continuing to grow. Sub-Saharan Africa leads the world with 25.3 million infected individuals with South-east Asia (5.8 million cases) the next area of concern. In South Africa alone there are an estimated 5 million people living with HIV/AIDS, most of whom are not receiving HAART.
It has been considered that the rate of CMVR is lower in Africa than in the United States, possibly related to the fact that, lacking effective therapy, patients in Africa may not live long enough to develop the very low CD4 cell counts (<50/cu.mm) that are associated with the development of CMV disease.1,2 Over the last 4 years we have, however, witnessed a steady increase in the number of patients presenting to our clinic with CMVR. This increase may be due partly to better management of tuberculosis and prophylaxis for Pneumocystis carinii pneumonia (PCP), which has meant longer survival of patients and lower CD4 cell counts, and partly to a greater awareness of the disease with earlier referral.
In 1996, when the first few cases of CMVR started presenting to our clinics, we were faced with a dilemma. How could we afford to treat this disease when numbers started to increase? The first few patients were treated with systemic ganciclovir (GCV), but the results were poor and the cost very high. The only option was repeated intravitreal injections of GCV as, theoretically, up to 250 patients could be treated with a single vial of GCV. The aim was preservation of vision, and patients understood that they would not be protected against systemic CMV disease or involvement of the other eye at a later stage. We have been treating all our CMVR patients in this manner since then. All their case notes were recently reviewed.
Patients and Methods
All patients presenting to our clinics with CMVR since April 1996 were treated with intravitreal GCV injections. Two patients were given oral GCV for a short period, but returned to intravitreal injections when both showed progression of their disease. The reasons for non-treatment were (a) patient refusal, (b) no potential for vision and (c) less than 3 clock hours of disease in zone III only (anterior to the equator). This last group was carefully watched and treatment initiated if the disease progressed into zone II, or extended beyond 3 clock hours in zone III, as the risk of retinal detachment significantly increases if more than 25% of the peripheral retina is involved.
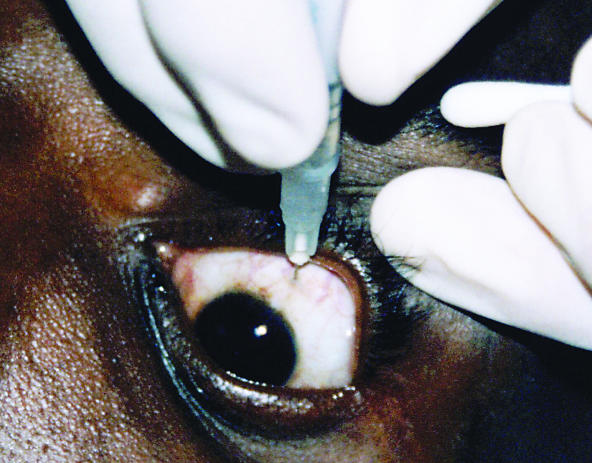
The procedure was performed in the outpatient clinic after written, informed consent was obtained. The GCV was reconstituted to a concentration of 25mg/ml using normal saline solution. A drop of local anaesthetic was instilled into the lower fornix, after which the eye was rinsed with a 5% povidone-iodine solution. A cotton-tipped applicator, soaked in local anaesthetic, was then held to the conjunctiva at the site of injection for 1 to 2 minutes. Using an insulin (1ml) syringe with a 30G needle, 2mg (0.08ml) of the GCV solution was injected into the vitreous, 4 mm posterior to the limbus superiorly (Figure 1). For the first 2 to 3 weeks, the patients returned bi-weekly for injections and, thereafter, on a weekly basis. (Further information is given in the ‘boxed’ appendix at the end of this article).
Fig. 1.
The injection is given 4mm posterior to the limbus
Photo: Linda Visser
INTRAVITREAL INJECTION OF GCV
-
Method of Preparation and Injection of GCV:
- One vial of ganciclovir (500mg) is reconstituted with 10 ml normal saline to a concentration of 50mg/ml. This is further diluted with normal saline (1:1) to a concentration of 25mg/ml (2mg/0.08ml).
- The injection is given with the patient lying down
- Fornices are rinsed with povidone-iodine solution
- Topical anaesthetic drops are applied
- A cotton-tipped applicator, soaked in topical anaesthetic, is held to the conjunctiva at the injection site for 1–2 minutes
- The injection is given 4mm behind the limbus superiorly with the patient looking down
- A 1ml syringe with a 30G (0.3×13mm) removable needle is used.
-
Price and Storage:
- One vial of ganciclovir (Cymevene, manufactured by Roche) costs between $20 and $30. We perform approximately 20 injections with 1 vial ($1 per injection), but theoretically 250 injections can be done (8c per injection)
-
Depending on the concentration of the ganciclovir, it has been reported to remain stable in a normal saline solution for between 12 hours (at 50mg/ml) and 35 days (at 5mg/ml).At 25mg/ml it seems to be stable for at least 72 hours.
- The manufacturer recommends that diluted solutions be kept refrigerated at 2–8 degrees Celsius and discarded after 24 hours as sterility cannot be guaranteed. We however discard the vial after 72 hours in order to use only 1 vial per week, as patients receive 2 injections per week during induction of treatment (2 weeks).
-
Exclusion Criteria
- No recoverable vision
- Less than 3 clock hours of disease in zone III
- No fundal view
-
Patients not prepared or able to come for regular injections.(* External eye disease, e.g., blepharitis is not an exclusion criterium, though this should be treated and the patient carefully watched.)
-
Safeguards and Training:
- The injection is only given by myself or an ophthalmic registrar/medical officer. I do not think that it should be given by someone who does not know the anatomy of the eye well
- I have taught a number of registrars and medical officers how to do the injections. It is fairly simple and anyone who has done any ocular surgery will be able to do it.
Linda Visser
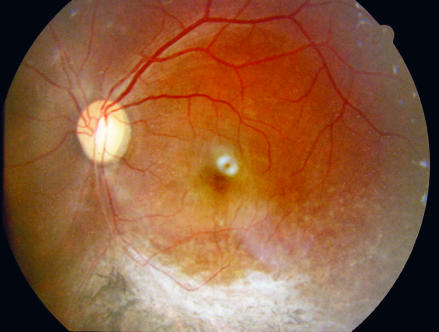
CMV retinitis
Photo: Linda Visser
Results
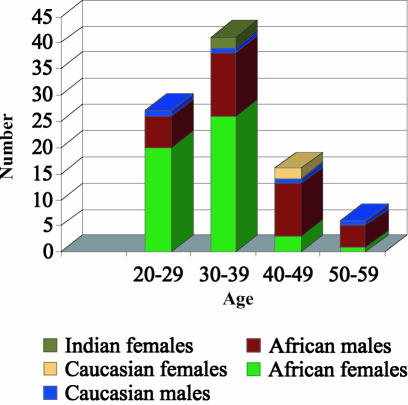
Between April 1996 and April 2003, 90 patients (123 eyes) were treated. A total of 1566 injections were given – 175 between April 1996 and December 1999 and 1391 between January 2000 and April 2003, clearly illustrating the rapid increase in numbers of patients presenting with CMVR over the last 3 years. All the patients were HIV positive. Only 15 patients were on anti-retroviral therapy at some point during their treatment (16.6%) and 30 patients (33.3%) were on cotrimoxazole prophylaxis for PCP. Tuberculosis was the most common other opportunistic infection in our patients, with 51 patients (56.6%) either concomitantly or previously infected. Patient demographics are shown in Figure 2.
Fig. 2.
Patient demographics
The highest incidence was seen in African females between the ages of 20 and 39 years. Most patients (75%) had bilateral disease at presentation. Of the 22 patients who presented with unilateral disease, only 2 (9%) developed CMVR in the contralateral eye after treatment had been initiated. To our knowledge, no patient developed systemic CMV disease.
Using only those eyes that had received 6 or more injections, the presenting visual acuity (VA) was compared to the final noted VA. The VA improved in 42 eyes (51%), remained unchanged in a further 12 (15%) and deteriorated in 28 (34%). In those eyes where the VA deteriorated, 23% lost 3 or fewer lines and only 11% lost 4 or more lines.
Progression, which is defined as the movement of disease by 750 microns over a 750 micron front or the development of a new lesion, did not occur when patients attended regularly for their injections. It was, however, seen in 10 patients:
4 patients had missed more than 3 consecutive injections due to illness
4 patients had been put on fortnightly injections and progressed after an average of 8 weeks
2 patients progressed while on oral treatment only.
Complications
Five vitreous haemorrhages, 3 of which were insignificant, cleared spontaneously within 2 weeks and were likely to have been the direct result of the injection. The other 2 haemorrhages were more severe, but occurred in patients who had retinal new vessels. One diabetic patient, who had new vessels at the disc had to have pan-retinal photocoagulation. The vessels regressed, the haemorrhage cleared spontaneously and did not recur after further injections. The other patient had peripheral new vessels following chorioretinitis/retinal vasculitis of unknown cause (though TB was suspected). As the haemorrhage was dense, the patient had a vitrectomy and sector retinal photocoagulation.
There were 6 cataracts in 5 patients, 4 of whom were over 45 years of age and were on HAART and thus had chronic uveitis. The other cataract was found in the patient who had had a vitrectomy for vitreous haemorrhage. None were caused by direct injury to the lens during injection.
One patient sustained a small hyphaema due to an iris root injury when she jerked her head away just as the injection was about to be given – it was her first injection.The hyphaema cleared within a day and she has been much more compliant since then.
As mentioned, 4 patients who were on HAART developed chronic uveitis - possibly related to immune recovery.
There were 3 retinal detachments (RDs), but all occurred within 3 weeks of presentation in patients with more than 50% of the retina involved (high risk for RD). No RDs were seen once the retina started to scar down.
Sadly, we had 4 cases of endophthalmitis, 3 of which occurred on the same day.
Discussion
CMVR is increasing in South Africa, possibly due to better management of patients and prophylaxis for other opportunistic diseases. HAART, which is becoming available to more people, is by far the most valuable weapon in our fight against CMVR. Systemic anti-CMV drugs are very expensive, have many side effects and are generally not as effective as local therapy. The GCV implant is too expensive and fomivirsen is not readily available. Repeated intravitreal injections of GCV have been shown to be very effective, relatively safe and extremely affordable. The only drawback is that it is time-consuming and labour-intensive. Some would argue that local therapy alone does not offer protection against contralateral eye or systemic involvement. However, our figure of 9% subsequent infection compares well with the GCV-FOS trial done in America prior to HAART, which showed a 17% risk of fellow eye disease in patients on either systemic GCV or foscarnet.3
A retrospective review of 648 cases of CMVR seen at Johns Hopkins University School of Medicine, Baltimore, showed the one year cumulative incidence of loss of 3, 6 and 10 lines of VA in their patients to be 42%, 30% and 23% respectively.4 Many of these patients had been on HAART. In our study, 23% of patients lost 3 or fewer lines of VA and only 11% lost more than 3 lines and very few patients were on HAART. HAART did not seem to make a difference to the visual outcome, but what was of great importance to the patients was the fact that, for those on HAART, GCV injections could be discontinued once immunity was re-established.
If HAART became more readily available and a cheaper GCV implant could be produced for the developing world, our problems might be something of the past. However, until such time we will continue to treat our patients in this manner.
References
- 1.Carmichael T R, Sher R. Screening for cytomegalovirus retinitis at an AIDS clinic. SA Medical Journal. 2002;92:445–446. [PubMed] [Google Scholar]
- 2.Beare N A, Kublin J G, Lewis D K, Schijffelen M J, Peters R P, Joaki G, Kumwenda J, Zijlstra E E. Ocular disease in patients with tuberculosis and HIV presenting with fever in Africa. Br J Ophthalmol. 2002;86:1076–1079. doi: 10.1136/bjo.86.10.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Studies of the Ocular Complications of AIDS Research Group, in collaboration with the AIDS Clinical Trials Group. Foscarnet-ganciclovir cytomegalovirus retinitis trial 4: visual outcomes. Ophthalmology. 1994;101:1250–1261. [PubMed] [Google Scholar]
- 4.Kempen J H, Jabs D A, Wilson L A, Dunn J P, West S K, Tonascia J A. Risk of vision loss in patients with cytomegalovirus retinitis and the acquired immunodeficiency syndrome. Arch Ophthalmol. 2003;121:466–475. doi: 10.1001/archopht.121.4.466. [DOI] [PubMed] [Google Scholar]