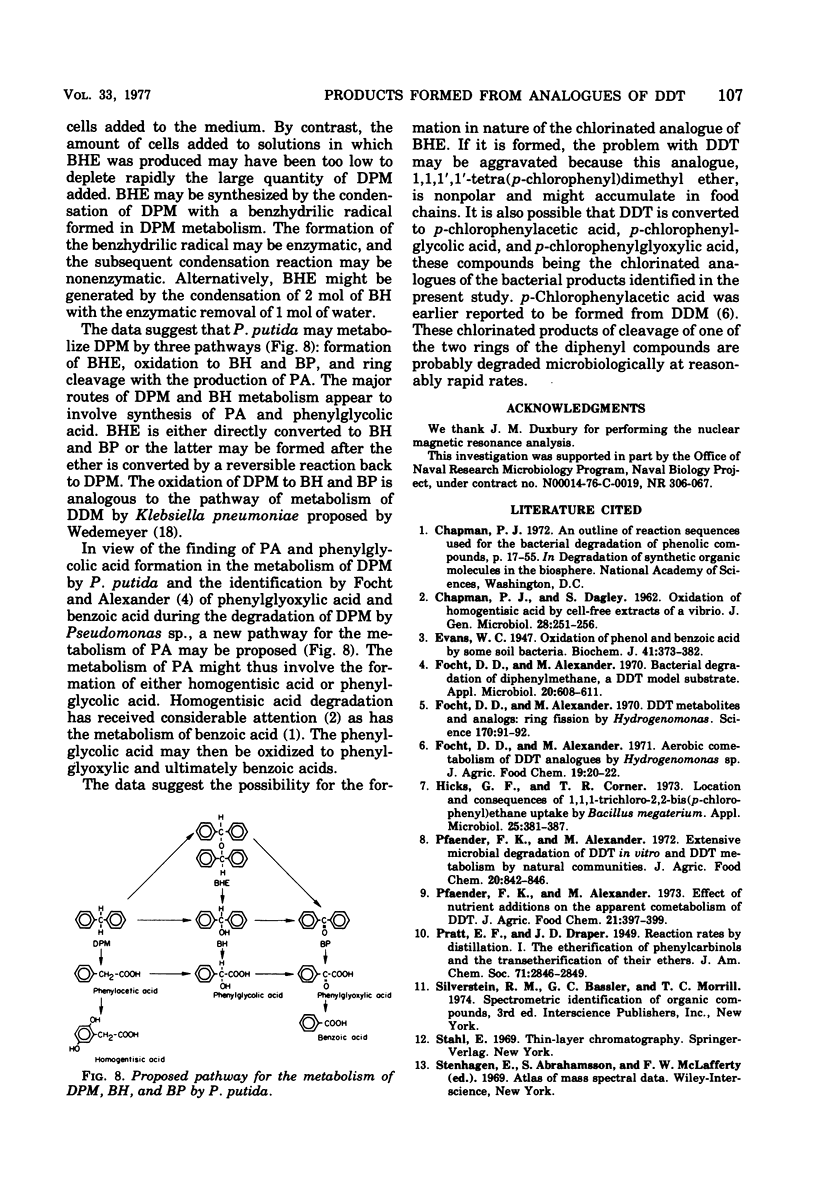
Abstract
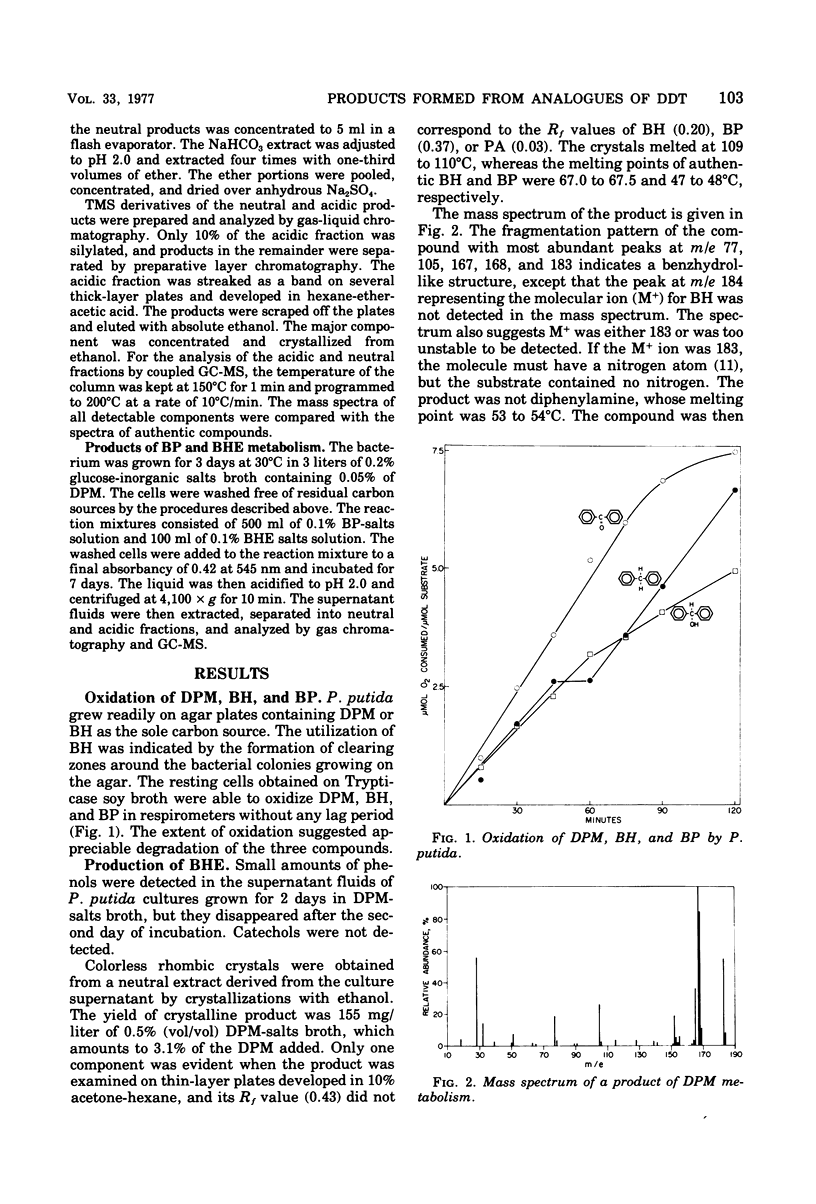
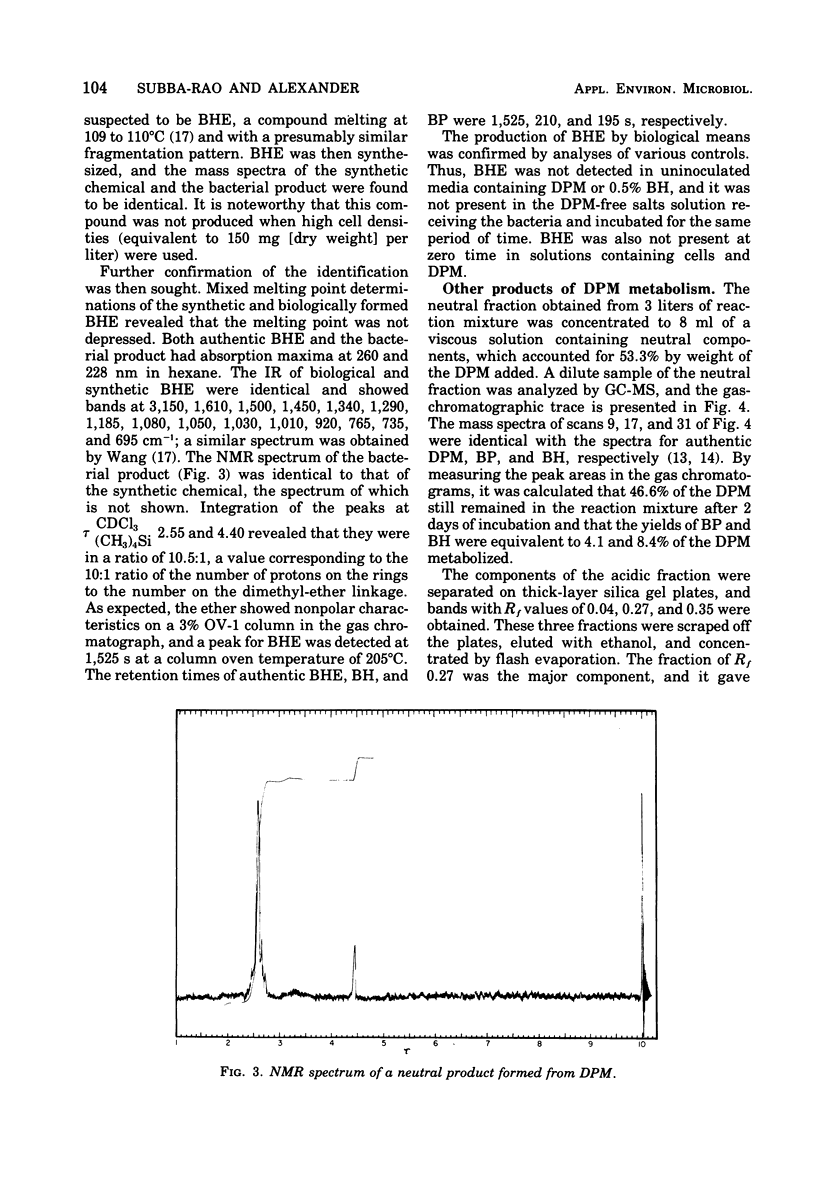
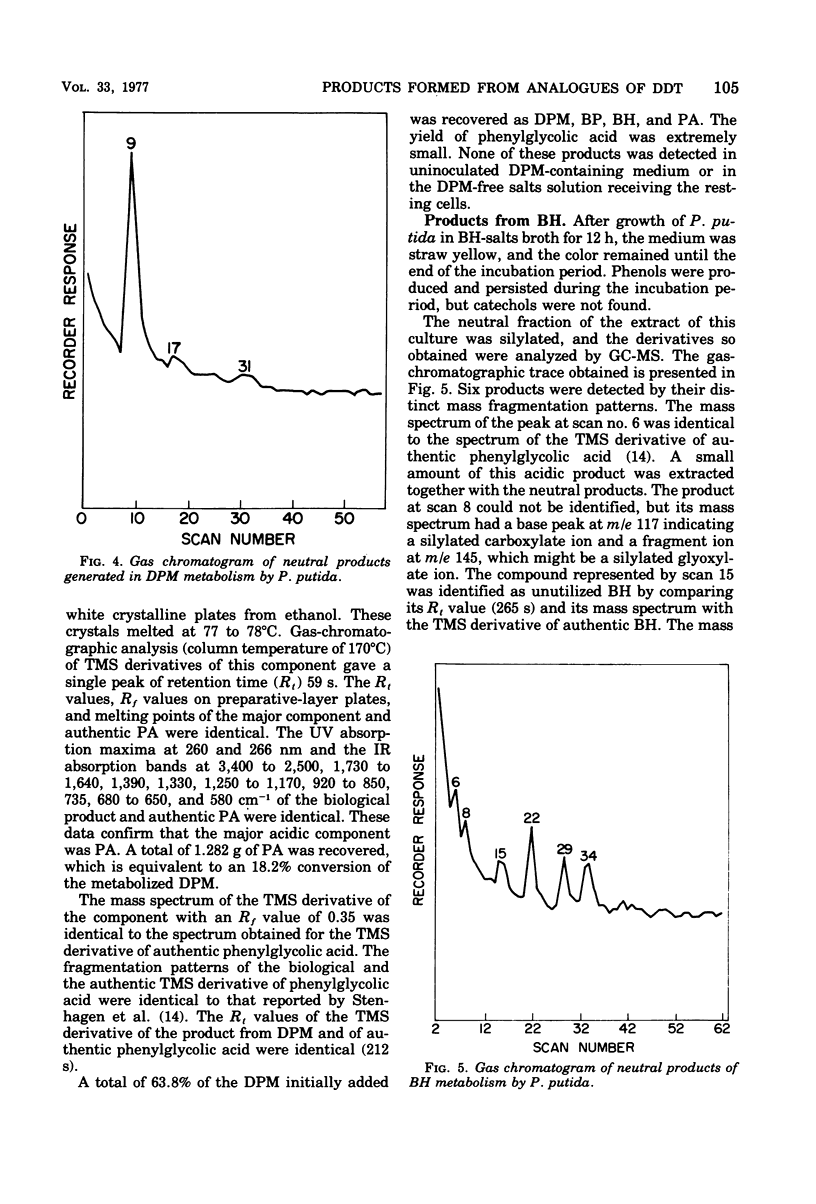
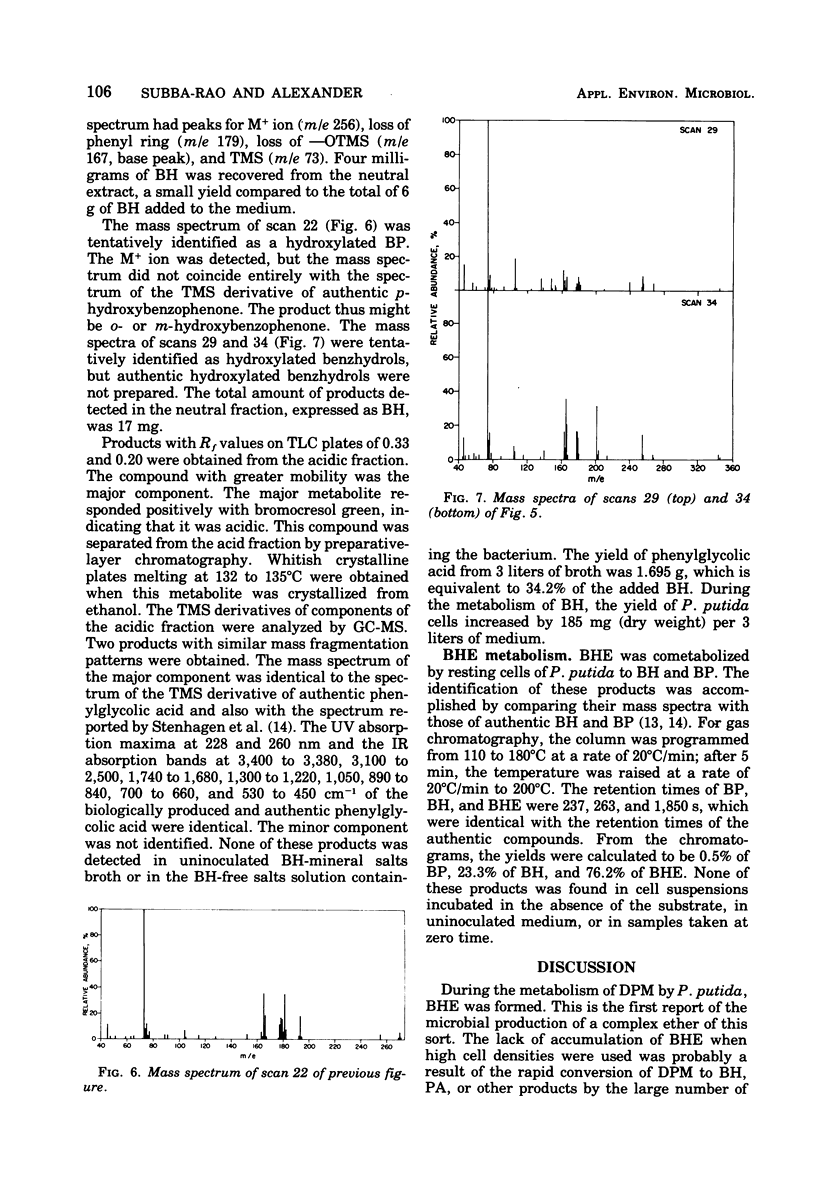
Cultures of Pseudomonas putida growing in solutions with diphenylmethane as sole carbon source formed 1,1,1′,1′-tetraphenyldimethyl ether. The product was identified by gas chromatography, mass spectrometry, and infrared and nuclear magnetic resonance spectrometry. The formation of benzophenone, benzhydrol, and phenylglycolic acid was established by gas chromatography and mass spectrometry. Similar techniques also revealed that phenylacetic acid was a major metabolite. Resting cell suspensions converted benzhydrol to phenyl-glycolic acid and products tentatively identified as hydroxybenzhydrols and a hydroxybenzophenone. Cell suspensions of the bacterium also converted the tetraphenyldimethyl ether to benzhydrol and benzophenone. Possible pathways for the degradation of these analogues of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) metabolites are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPMAN P. J., DAGLEY S. Oxidation of homogentistic acid by cell-free extracts of a vibrio. J Gen Microbiol. 1962 Jun;28:251–256. doi: 10.1099/00221287-28-2-251. [DOI] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. Aerobic cometabolism of DDT analogues by Hydrogenomonas sp. J Agric Food Chem. 1971 Jan-Feb;19(1):20–22. doi: 10.1021/jf60173a042. [DOI] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. Bacterial degradation of diphenylmethane, a DDT model substrate. Appl Microbiol. 1970 Oct;20(4):608–611. doi: 10.1128/am.20.4.608-611.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. DDT metabolites and analogs: ring fission by Hydrogenomonas. Science. 1970 Oct 2;170(3953):91–92. doi: 10.1126/science.170.3953.91. [DOI] [PubMed] [Google Scholar]
- Hicks G. F., Jr, Corner T. R. Location and consequences of 1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane uptake by Bacillus megaterium. Appl Microbiol. 1973 Mar;25(3):381–387. doi: 10.1128/am.25.3.381-387.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender F. K., Alexander M. Effect of nutrient additions on the apparent cometabolism of DDT. J Agric Food Chem. 1973 May-Jun;21(3):397–399. doi: 10.1021/jf60187a046. [DOI] [PubMed] [Google Scholar]
- Pfaender F. K., Alexander M. Extensive microbial degradation of DDT in vitro and DDT metabolism by natural communities. J Agric Food Chem. 1972 Jul-Aug;20(4):842–846. doi: 10.1021/jf60182a045. [DOI] [PubMed] [Google Scholar]
- Wedemeyer G. Biodegradation of Dichlorodiphenyltrichloroethane: Intermediates in Dichlorodiphenylacetic Acid Metabolism by Aerobacter aerogenes. Appl Microbiol. 1967 Nov;15(6):1494–1495. doi: 10.1128/am.15.6.1494-1495.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


