Onchocerciasis, also known as ‘river blindness’, is an insect-borne disease, caused by a nematode worm, Onchorcerca volvulus. It is the world's second leading infectious cause of blindness. In most of these countries it constitutes a public health problem and a serious obstacle to socio-economic development.
Disease Prevalence and Burden
About 125 million people world-wide are estimated at risk of onchocerciasis, and, of these, 96% are in Africa.
Of the 37 countries where the disease is endemic, 30 are in sub-Saharan Africa, six are in the Americas and one is in the Arabian Peninsula.
A total of 18 million people are infected with the disease, of whom 99% live in Africa and at least one million are either blind or severely visually disabled. To these are added each year an estimated 40,000 new blind.
As the name ‘river blindness’ suggests, onchocerciasis is essentially a focal disease. However, where it exists, its impact on affected communities may be quite extensive and devastating. Thus, in many hyperendemic areas with blinding onchocerciasis, almost every person will be infected, and half of the population will be blinded by the disease before they die. Once blind, affected individuals have a life expectancy of only one third that of the sighted and most die within 10 years.
Recent studies in Ethiopia, Nigeria and Sudan have also shown that onchocerciasis is responsible for poor school performance and a higher drop out rate among infected children (due to itching, lack of sleep, etc.), while low productivity, low income and higher health-related costs are found among infected adults.
Disease Transmission
The parasite. Onchocerca volvulus, the causal agent of onchocerciasis is one of a large group of nematodes. The adult worms live encysted in fibrous nodules. Each nodule contains between 2–3 female worms lying in a twisted, tangled mass, hence the term volvulus. Adult female worms have a life span of 8 to 10 years but may live up to 15 years, during which time each releases millions of first-stage larvae, also known as microfilariae. In hyperendemic areas, the total microfilaria load in the body of affected individuals may be as high as 150 million.
The vector. Onchocerciasis is transmitted from one individual to another by a black fly of the genus Simulium. The blackfly larvae require well-oxygenated water to mature, and eggs are laid in rapids in fast flowing rivers and streams. Female black flies require a blood meal to produce/lay eggs, and it is during this meal that they may transmit or receive the onchocercal infection.
Cycle of infection. Microfilariae enter a female blackfly when she bites an infected person. A small percentage of these reach the insect's thoracic muscles where after several moults, they become third-stage infective larvae. They then migrate to the insect's salivary glands and are ready to be transferred during the next blood meal.
After entering the skin of the human host through the bite of an infected blackfly, the infective larvae (usually two to six) migrate through the subcutaneous tissues. Here, over the next 12 months, each larva will mature into an adult male or female worm. Before a heavy load of adult worms and pathogenic microfilariae builds up in the human host, this sequence has to be repeated many times over, and many years of exposure are usually required.
Clinical Manifestations
The people most at risk from onchocerciasis are those who for reasons of occupation (e.g., fishermen, farmers, sand diggers) have spent long hours or live nearer to the breeding sites. Early manifestations of the disease in infected persons usually appear one to three years after the injection of infective larvae. Nearly all the lesions of onchocerciasis including those in the eye, are directly or indirectly related to local death of microfilariae. Generally, live microfilariae stimulate very little inflammatory response and the mechanisms that protect them from the host's immune response are still largely unknown.
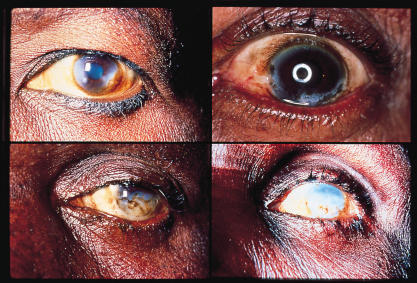
Sclerosing keratitis in onchocerciasis
Photos: Ian Murdoch & Allen Foster
The clinical features of onchocerciasis may be divided into two main groups: ocular and non-ocular, as summarised in Boxes 1 and 2.
Box 1: Non-Ocular Manifestations
Pruritus: often severe and unrelenting
Nodules: subcutaneous, painless, typically found around bony prominences (iliac crest, greater trochanters, ribs, knees, coccyx and skull)
Severe, disfiguring skin disease: may lead to the distress of social stigma, psychological and sleep disorders
Lymphatic: lymphadenopathy, hanging groin
Unproven but suspected associations: hyposexual dwarfism, higher prevalence of epilepsy
Box 2: Ocular Manifestations
Anterior segment
Live microfilariae in anterior chamber (AC)
Punctate keratitis, leading on to sclerosing keratitis
Early uveitis, leading on to chronic uveitis
Posterior segment
Choroido-retinitis, leading on to choroido-retinal atrophy or optic nerve atrophy
Acute optic neuritis, leading on to optic atrophy
Others
Night blindness
Visual field loss and constriction
Irreversible blindness
Control of Onchocerciasis
The past ten years have seen a rapid and remarkable expansion of onchocerciasis control activities worldwide, thanks to joint efforts and support from WHO and other UN agencies, the World Bank and a growing coalition of Non-Governmental and Development Organizations (NGDOs). These efforts are coordinated by three major regional programmes, one in Central and Latin America, the Onchocerciasis Control Programme of the Americas (OEPA); and two in Africa, the Onchocerciasis Control Programme (OCP), and the African Programme for Onchocerciasis Control (APOC). Together these three regional programmes cover more than 99% of all endemic populations and all but one (Yemen) endemic countries. (Please see the Editorial by Dr Bjorn Thylefors).
Strategy Options for the Control of Onchocerciasis
The control of onchocerciasis today is based essentially on two strategies: Similium vector control and large-scale chemotherapy with ivermectin. Each may be used alone or in combination.
Vector control. This is the chief strategy used in West Africa by the Onchocerciasis Control Programme (OCP) since 1974. The main goal in vector control is to interrupt transmission of O. volvulus by regular aerial spraying of all Simulium larval breeding sites, and to maintain this for at least 14 years until the infection has died out in human populations. This strategy, used alone at the beginning and now in combination with ivermectin, has been highly effective: onchocerciasis has been virtually eliminated in the original seven OCP countries, and progress elsewhere in the programme area is so advanced as to justify the closing down of OCP in 2002. For reasons of cost and operational feasibility, vector control could not be applied or extended to other endemic countries outside the OCP area of operation.
Chemotherapy. Ivermectin is the only chemotherapeutic agent recommended for use against onchocerciasis. Its mass distribution constitutes the main strategy for the other two regional programmes, APOC and OEPA. It is a semisynthetic, macrocyclic, lactone antibiotic widely used in the field of veterinary medicine against a wide range of animal parasites. It was developed during the 1980s. In 1987, the manufacturers, Merck & Co., made the generous decision to donate ivermectin free for the treatment of human onchocerciasis, ‘to as many as needed and for as long as required’. Its main characteristics can be summarised as follows:
It is a microfilaricide, with a very wide therapeutic range (150–800 micrograms/kg)
It is highly attractive and popular in endemic communities for its many other beneficial effects on intestinal worms, scabies, head lice, and for its supposed enhancing effect on libido
Given at the recommended single dose of 150μ/kg, it is effective for up to a year.
When given to the largest sections of affected communities, it may significantly reduce disease transmission
However, because ivermectin has no demonstrable direct effect on the adult worm, it must be given repeatedly for up to 12–15 years, i.e., the time it takes for most adult worms to die.
Current Uses of Ivermectin in Onchocerciasis Control
There are two main uses of ivermectin in the treatment of onchocerciasis: passive or clinic based, and active, as in large scale or mass treatment of entire communities.
Passive or clinic-based treatment. This is the form of treatment available to all those seeking medical treatment and in whom a clinical diagnosis of onchocerciasis has been made in a hospital or health centre. It is directed primarily to infected individuals and is the main method of treatment in hypoendemic areas where the risk of blindness or severe skin disease is virtually non-existent.
Community mass treatment. Also known as community-wide treatment, this is the method of choice in meso- and hyperendemic areas of onchocerciasis, i.e., where onchocerciasis is considered a public health problem. In these areas ivermectin is given once a year for at least 14 years, as recommended in Table 1, to all members of the community except for the exclusions defined in Box 3.
Table 1.
Recommended Doses of Ivermectin in Mass Treatment
| Weight (kg) | Height (cm) | No. of Tablets (3 mg) |
|---|---|---|
| 15–25 | 90–119 | 1 |
| 26–44 | 120–140 | 2 |
| 45–64 | 141–158 | 3 |
| 65 or more | 159 or more | 4 |
Box 3: Exclusion Criteria for Ivermectin Treatement
Children under 5 years (age), or Less than 15 kg (weight), or Less than 90 cm (height)
Pregnant women
Lactating mothers of infants less than one week old
Severely ill persons
Use with extreme caution in areas co-endemic with Loa loa
Over the years mass treatment has evolved from mobile strategies used in the early days following ivermectin donation to various forms of community-based treatment. In nearly all cases, these changes have been dictated by the need to reduce operation costs, increase treatment coverage and maximise programme impact on affected communities. The latest and most widely used of these community-based strategies is known as Community Directed Treatment with Ivermectin (CDTI). With this method considerable efforts are made to involve affected communities themselves in the planning, implementation and monitoring of treatment activities. CDTI is the preferred and official method used throughout Africa by both OCP and APOC.
Table 2 is a summary of current uses of ivermectin in onchocerciasis control, based on endemicity levels.
Table 2.
Treatment Approaches Based on Endemicity Levels
| Hyper-endemic (40+% nodule carriers) | Mass treatment (CDTI) |
| Meso-endemic (20–39% nodule carriers) | |
| Hypo-endemic (<20% nodule carriers) | Clinic-based treatment |
| Non endemic | No treatment |
Ivermectin treatment greatly reduces transmission of the parasite, but does not halt it. As and the adult worm may live for as long as 14–15 years, annual large-scale treatment will therefore have to continue for a very long time. Recent predictions with a simulation model have indicated that at coverage levels of around 65%, annual treatment may have to continue for up to two decades. The main challenge facing ivermectin-based control, therefore, is to develop and implement simple methods of ivermectin delivery which can be sustained by the communities themselves. Hence the attractiveness of CDTI.
The risk of resistance to ivermectin is remote within the time frame of the proposed Programme. However, the history of parasite disease control based on chemotherapy suggests that a cautious approach should be adopted. Recent model simulations and molecular biological studies indicate that resistance could become a problem over a twenty- to thirty-year time period, despite the long generation time of O. volvulus. It is important, therefore, to continue the development of alternative drugs for the treatment of onchocerciasis.
Macrofilaricide: Moxidectin
The development of a macrofilaricide drug is currently being undertaken through the MACROFIL project, a WHO based project which aims to develop a macrofilaricide, i.e., one which kills the adult worms.
Of the many candidate drug compounds that been tested and identified so far, moxidectin, has shown to be the most promising.
Final results of moxidectin trials in animal models were reviewed by WHO in March 2000. These pre-clinical studies have shown it to fulfil many of the criteria for a potential macrofilaricide: easy to use, safe and effective.
At present moxidectin is only available in veterinary formulations, but plans are under way to start clinical trials in humans.
References
- 1.Report of WHO Expert Committee, Technical Report Series No. 852. Geneva, World Health Organization, 1995. [PubMed]
- 2.Epidemiology of Onchocerciasis. Report of WHO Expert Committee, Technical Report Series No. 752. Geneva, World Health Organization, 1987.
- 3.Duke BOL. Onchocerciasis. In: Johnson GJ, Minassian DC, Weale R, editors. London: The Epidemiology of Eye Disease; 1998. pp. 227–247. [Google Scholar]
- 4.Buck AA. Onchocerciasis: Symptomatology, Pathology, Diagnosis. Geneva, World Health Organization, 1974.
- 5.Benton B. Economic impact of onchocerciasis control thrpugh the African Programme for Onchocerciasis Control: An overview. Ann Trop Med Parasitol. 1998;92(1):S33–S39. doi: 10.1080/00034989859537. [DOI] [PubMed] [Google Scholar]
- 6.Etya'ale DE. Mectizan as a stimulus for the development of novel partnerships: the international organization's perspective. Ann Trop Med Parasitol. 1998;92(1):S73–S77. [PubMed] [Google Scholar]