Introduction
Corneal scar is a significant cause of visual impairment and blindness in the developing world. Corneal infections are responsible for a large proportion of this scarring. A review of the data on indications for corneal transplantation in the developing world revealed that corneal scar was the most common indication (28.1%), of which keratitis accounted for 50.5%. Besides this, about 12.2% of all grafts were done for active infectious keratitis.1 Thus suppurative keratitis and its complications constitute important causes of ocular morbidity, particularly in the developing world.
Almost any organism can invade the corneal stroma if the normal corneal defence mechanisms, i.e., lids, tear film and corneal epithelium are compromised. While viral infections are the leading cause of corneal ulcer in the developed nations (with Acanthamoeba infection in contact lens wearers), bacteria, fungi and Acanthamoebae are important aetiological agents in the developing world. The spectrum of corneal pathogens shows a wide geographical variation. At L V Prasad Eye Institute, Hyderabad, 71.9% of all cases of ulcerative keratitis were culture positive. Of the culture positive cases 63.9% were bacterial, 33% were fungal, 2.1% were parasitic, and 6.2% were due to mixed infection. Various organisms isolated from cases of infectious keratitis are shown in Table 1.
Table 1.
Various Isolates from Cases of Infectious Keratitis. L V Prasad Eye Institute: January 1991 – December 1998 (n=2655)
| Bacteria: n=1689 | |
| Gram positive cocci | |
| Staphylococcus epidermidis | 32.4% |
| Staphylococcus aureus | 7.6% |
| Other staphylococci | 4.0% |
| Streptococcus pneumoniae | 13.1% |
| α-haemolytic streptococci | 5.3% |
| Other streptococci & micrococci | 1.6% |
| Gram positive bacilli | |
| Corynebacterium | 13.9% |
| Bacillus | 1.2% |
| Nocardia | 1.7% |
| Mycobacterium | 0.4% |
| Propionibacterium | 1.2% |
| Gram negative bacilli | |
| Pseudomonas | 11.1% |
| Enterobacteriaceae | 1.7% |
| Moraxella | 1.4% |
| Aeromonas | 0.4% |
| Acinetobacter | 0.7% |
| Haemophilus | 0.8% |
| Fungi: n=893 | |
| Aspergillus | 33.0% |
| Fusarium | 35.1% |
| Dematiaceous fungi | 14.4% |
| Other hyaline fungi | 16.4% |
| Candida | 1.0% |
| Parasites: n=73 | |
| Acanthamoeba | 100% |
In this article we focus on the diagnosis and management of suppurative corneal ulcer.
Diagnosis
A detailed history and thorough clinical examination using the slit-lamp biomicroscope are important steps in the diagnosis of corneal ulcer. Although clinical signs may be insufficient to confirm infection, a break in the continuity of the epithelium associated with underlying stromal infiltrate should be considered infectious unless proved otherwise. Similarly, there are no distinctive or exclusive signs to identify the responsible organisms, but clinical experience and careful slit-lamp examination can point toward a probable aetiological diagnosis in some cases. Gram-positive cocci typically cause localised round or oval ulceration with greyish white stromal infiltrates having distinct borders and minimal surrounding stromal haze. Keratitis due to gram-negative bacteria typically follows a rapid inflammatory destructive course characterised by dense stromal suppuration and hazy surrounding cornea with a ground glass appearance. Fungal keratitis is usually characterised by a dry raised slough, stromal infiltrate with feathery edges, satellite lesions, and a thick endothelial exudate. Acanthamoeba keratitis is characterised by epithelial irregularities with single or multiple stromal infiltrates in a classical ring-shaped configuration. Severe pain and radial keratoneuritis (i.e., inflammation of the corneal nerves, seen as a whitish outline of the corneal nerves) are also characteristics of Acanthamoeba infection.
Since the clinical appearance of suppurative keratitis depends on many variables, it is often difficult to arrive at an aetiological diagnosis based entirely on slit-lamp examination. For example, apart from Acanthamoeba keratitis (Fig. 1), the ring-shaped infiltrate can be seen in fungal keratitis (Fig. 2), HSV (herpes simplex) keratitis, and even in Pseudomonas keratitis. Similarly, Nocardia keratitis presents classically with multiple small white infiltrates arranged in a wreath pattern (Fig. 3), and it can have fine filaments extending into the surrounding cornea, similar to fungal keratitis. Pain out of proportion to the size of infiltrate and radial keratoneuritis, classically described for contact lens-related Acanthamoeba keratitis, is rarely experienced in non-contact lens related Acanthamoeba keratitis. The clinical picture is often confused if the lesions are peripheral, or advanced involving the entire cornea (Fig. 4). Laboratory investigations are therefore required if the causative organism is to be identified.
Fig. 1.
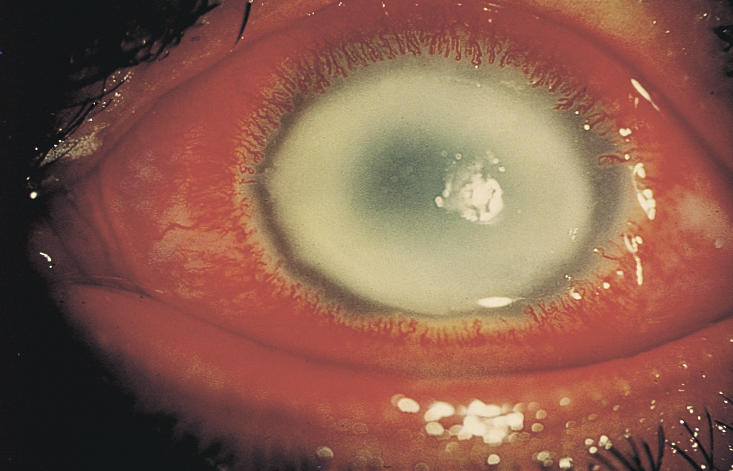
Ring infiltrate in Acanthamoeba keratitis
Photo: P Garg & G N Rao
Fig. 2.
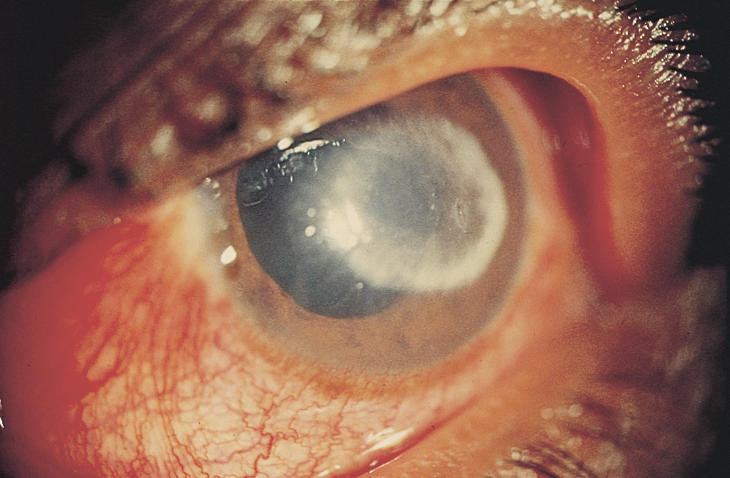
Ring infiltrate in fungal keratitis
Photo: P Garg & G N Rao
Fig. 3.
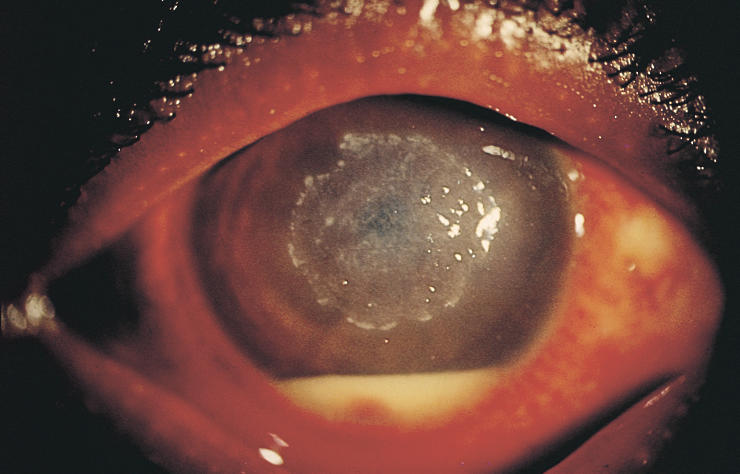
Nocardia keratitis with multiple pinhead infiltrates and hypopyon
Photo: P Garg & G N Rao
Fig. 4.
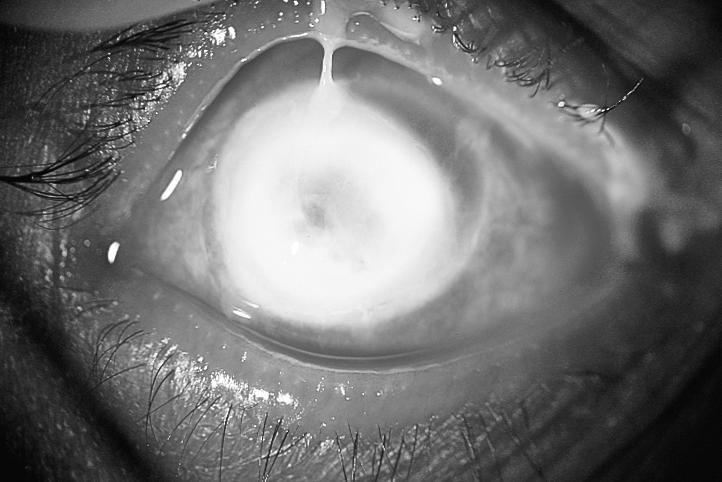
Corneal destruction due to Pseudomonas infection
Photo: P Garg & G N Rao
Laboratory Investigations
The laboratory procedures used in the diagnosis of infectious keratitis are based on:
direct visualisation of organisms in the material.
inoculation of material under appropriate conditions to allow multiplication of organisms.
Whenever a patient with infectious keratitis presents, after detailed clinical examination, corneal scrapings are taken under topical anaesthesia using a sterile No. 15 Bard Parker blade. Scrapings are taken from the edges and base of the ulcer (see Appendix). The material obtained is examined microscopically using Gram's (see Appendix) and Giemsa staining methods and potassium hydroxide 10% or calcofluor white preparation. Calcofluor white is a fluorescent dye and requires a fluorescent microscope. Lactophenol cotton blue stain may also be used which does not require a fluorescent microscope (see Appendix). The material is also inoculated on various solid and liquid media that facilitate the growth of bacteria, fungi, and Acanthamoeba. These include fresh blood agar, chocolate agar, Saburaud's dextrose agar (SDA), non-nutrient agar with an overlay of Escherichia coli, thioglycolate broth and brain heart infusion broth (Fig. 5). These media are incubated under appropriate atmospheric conditions and are examined daily for growth for at least seven days before a negative report is given. The growth on media is then identified and where appropriate is subjected to an antimicrobial susceptibility test. Microscopy using Gram's staining method and potassium hydroxide (KOH) preparation is simple and quick to perform and often gives useful information for initial medical management. Culture and sensitivity, on the other hand, require more sophisticated facilities.
Fig. 5.
Various culture media used in laboratory diagnosis of microbial keratitis
Photo: P Garg & G N Rao
Although the ophthalmic literature uniformly recommends that microbiological investigations must be performed in all cases of infectious keratitis, these procedures require investment of a certain amount of time and expense by the ophthalmologist, the patient and ultimately the medical system in general. A survey of community ophthalmologists in southern California showed that less than 20% of corneal ulcers were treated in accordance with textbook recommendations.2 Another study found that less than 4% cases required a change in initial antibiotic therapy based on an inadequate clinical response.3 It has also been documented that there may be poor correlation between in vitro antimicrobial sensitivity and in vivo clinical response. Consequently, there is some controversy over the routine use of microbiological investigations (including antimicrobial sensitivity testing) in the management of suppurative keratitis. Based on the experience gained at the L V Prasad Eye Institute and a relatively higher incidence of fungal keratitis (33%) in the tropical climate, we are of the opinion that microscopic examination of corneal scrapings using Gram's staining techniques and KOH (10%) preparation can provide useful guidance for initial therapy in a case of suppurative keratitis.
Treatment
When treating a patient with suppurative keratitis the clinician has 3 management options:
Complete microbiological work-up of all ulcers, followed by initial therapy based on the smear results;
Empirical therapy (based on previous clinical experience) with one or more commercially available broad spectrum antimicrobial agents; or
Microbiology work-up of severe ulcers where the history or clinical findings suggest an atypical non-bacterial pathogen.
It is clear that option 1 is the best approach for the tertiary referral practice, because most of the ulcers are severe or caused by unusual or resistant organisms that have failed to respond to initial therapy. However, there is a lot of confusion regarding the best option for community ophthalmologists. A large proportion of suppurative keratitis is caused by bacteria (64%), most of which are sensitive to broad spectrum antibiotics. It is reasonable, therefore, to assume that in small lesions that are away from the visual axis and not associated with risk factors for unusual organisms, initial treatment may be started with a broad spectrum antibiotic at frequent intervals. These patients, however, need close daily follow up to make sure the lesion is improving. At the earliest evidence of deterioration the ulcer should be subjected to a detailed microbiology work-up or referred to a centre where such facilities exist.
Treatment with a commercially available antibiotic that has a broad spectrum of activity against gram-negative and gram-positive organisms, such as ciprofloxacin or ofloxacin, seems to be the least expensive first approach. However, there is a risk of development of resistance particularly with ciprofloxacin.
Microbiological investigations should always be done for the following cases.
Severe ulcers (a rapidly progressing infiltrate which is more than 6mm in diameter or involves deeper stroma or associated with imminent or actual perforation).
Cases where history and clinical examination suggest unusual non-bacterial pathogens.
Initial treatment in these cases should be based on the microscopic examination.
Initial treatment in fungal keratitis is usually started with natamycin (5%) suspension administered half hourly. Various antifungal agents used in the treatment of keratitis are shown in Table 2.
Table 2.
Antifungal Agents used in Keratitis
| Polyenes |
| Nystatin |
| Amphotericin B |
| Natamycin |
| Pyrimidines |
| Flucytosine |
| Imidazoles |
| Clotrimazole |
| Miconazole |
| Ketoconazole |
| Fluconazole |
| Itraconazole |
For Acanthamoeba keratitis, treatment is usually started with polyhexamethylene biguanide (PHMB) 0.02% or chlorhexidine 0.02% (Table 3). Antifungal and anti-Acanthamoeba therapy is started only when microbiological evidences exists. Modification of therapy is primarily based on clinical response to initial therapy and is guided by the results of culture and sensitivity tests.
Table 3.
Anti-Acanthamoeba Agents used in Keratitis
| Antiseptic biocides |
| Chlorhexidine |
| PHMB |
| Aminoglycosides |
| Neomycin |
| Paromomycin |
| Diamidines |
| Dibromopropamidine |
| Hexamidine |
Supplementary Treatment
Cycloplegic agents such as atropine sulphate 1%, homatropine 1% or cyclopentolate 1% instilled three times a day reduce ciliary spasm and produce mydriasis, thereby relieving pain and preventing synechiae formation. Anti-glaucoma agents are used when intraocular pressure is high.
If required, oral analgesics for pain may be used.
The role of topical corticosteroids in the management of suppurative keratitis is controversial and hence they are best avoided.
Simple debridement of necrotic debris in conjunction with intensive topical therapy may help facilitate drug penetration especially of anti-fungal agents.
Tissue adhesive using N-butyl cyanoacrylate with a bandage contact lens is useful in cases with marked thinning or perforation less than 2mm.
Penetrating keratoplasty is performed in cases with advanced disease at presentation where there is no response to medical therapy or when a large perforation is present.
Prevention
Although not always a preventable disease, certain steps may help reduce the potentially severe consequences of suppurative keratitis.
Community awareness of risk factors for suppurative keratitis such as minor trauma and the use of contaminated traditional eye solutions in the eye
Early recognition and institution of appropriate therapy by community health workers or ophthalmologists
Prompt referral of advanced cases to tertiary eye care centres
Suppurative keratitis is a sight-threatening disorder. Early clinical suspicion, rational use of laboratory diagnostic procedures and appropriate therapy can go a long way towards reducing ocular damage from this disorder.
References
- 1.Dandona L, Krishnan R, Janarathanan M, et al. Indications for penetrating keratoplasty in India. Indian J Ophthalmol. 1997;45:163–8. [PubMed] [Google Scholar]
- 2.McDonnell PJ, Nobe J, Gauderman WJ, et al. Community care of corneal ulcers. Am J Ophthalmol. 1992;114:531–8. doi: 10.1016/s0002-9394(14)74479-4. [DOI] [PubMed] [Google Scholar]
- 3.McLeod SD, Kolahdouz-Isfahani A, Rosamian K, et al. The role of smears, cultures and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103:23–8. doi: 10.1016/s0161-6420(96)30738-0. [DOI] [PubMed] [Google Scholar]


