Abstract
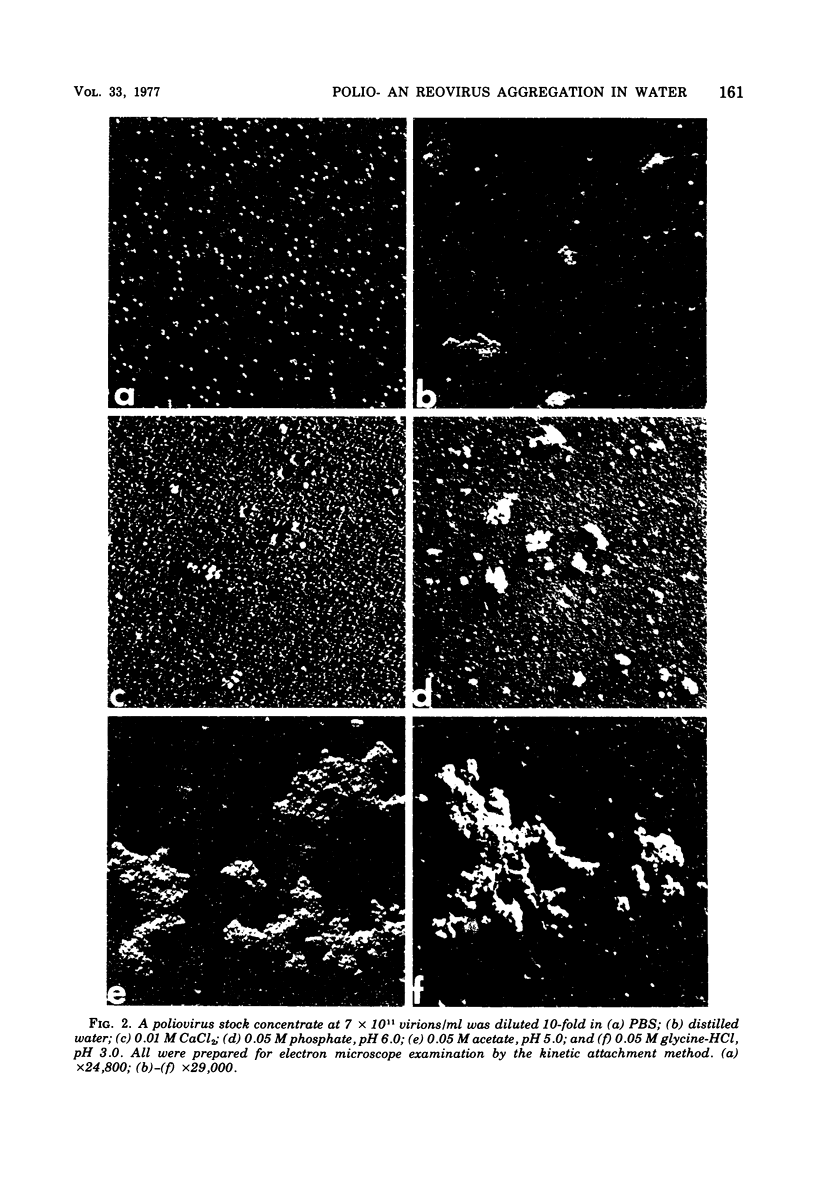
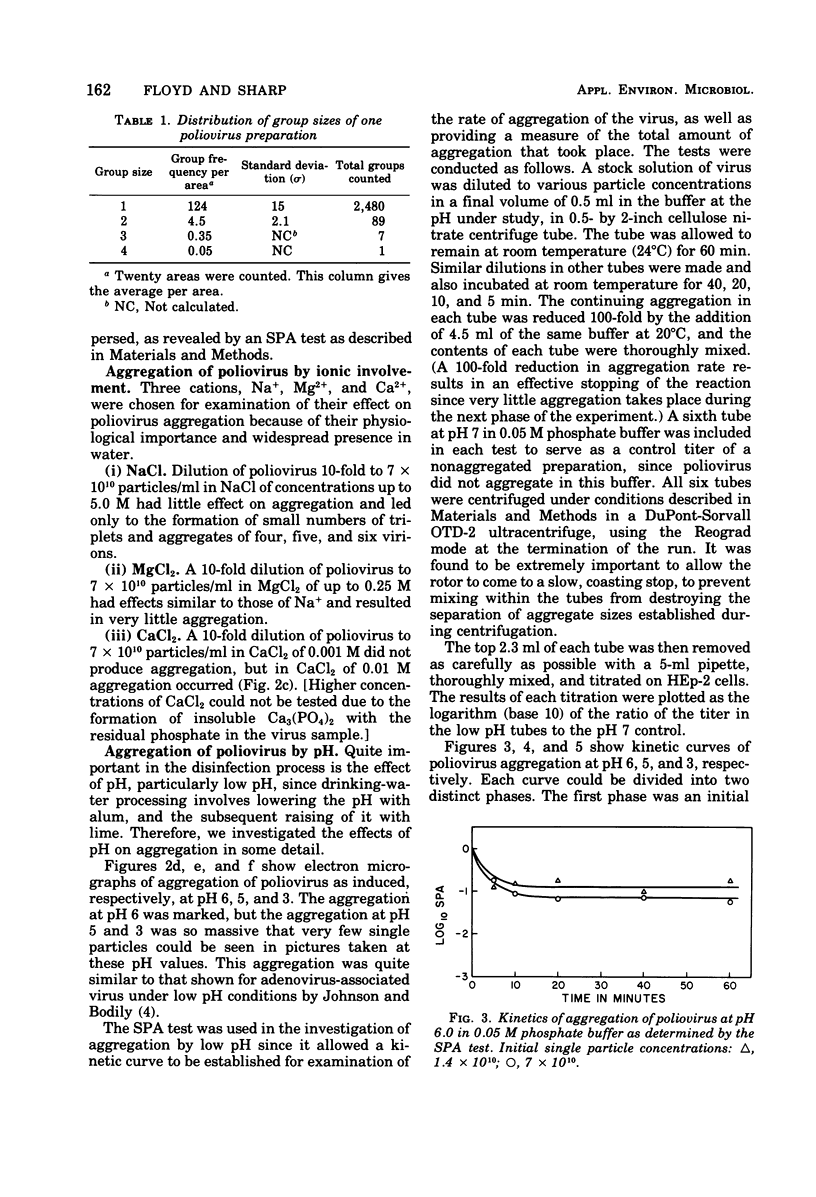
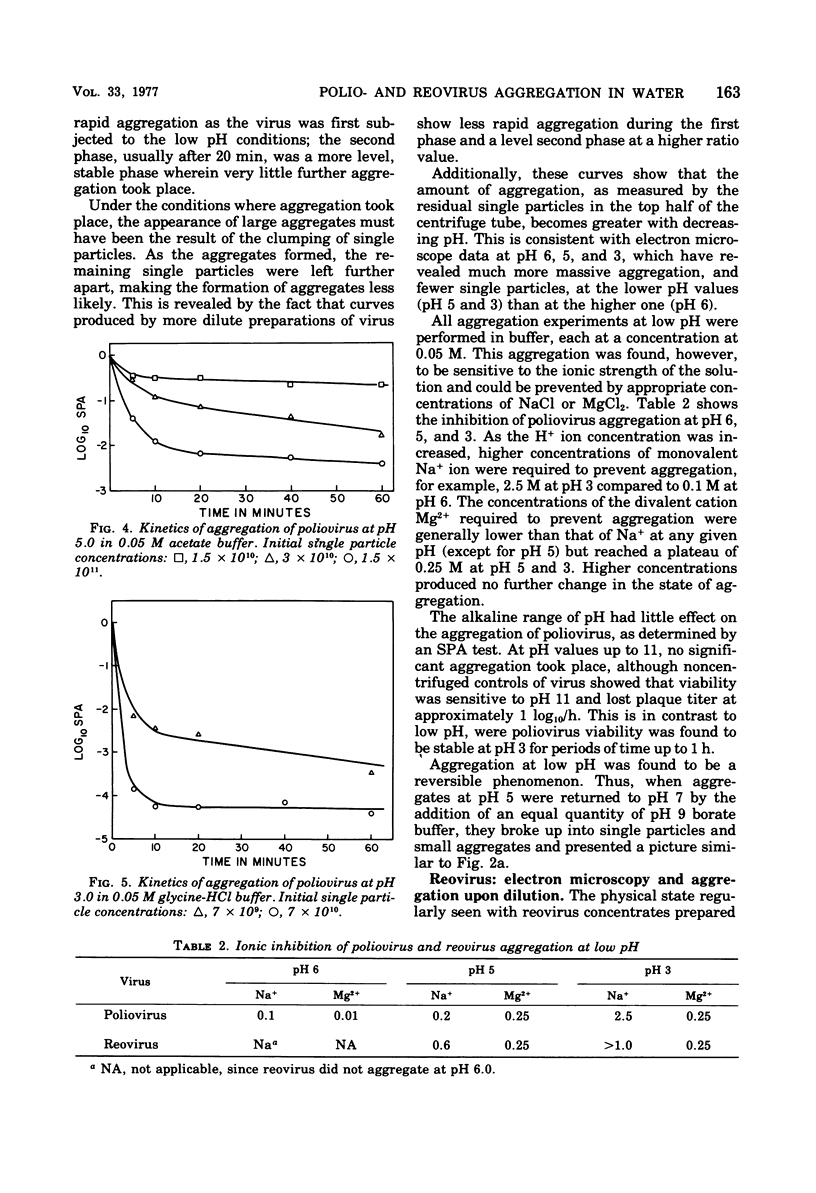
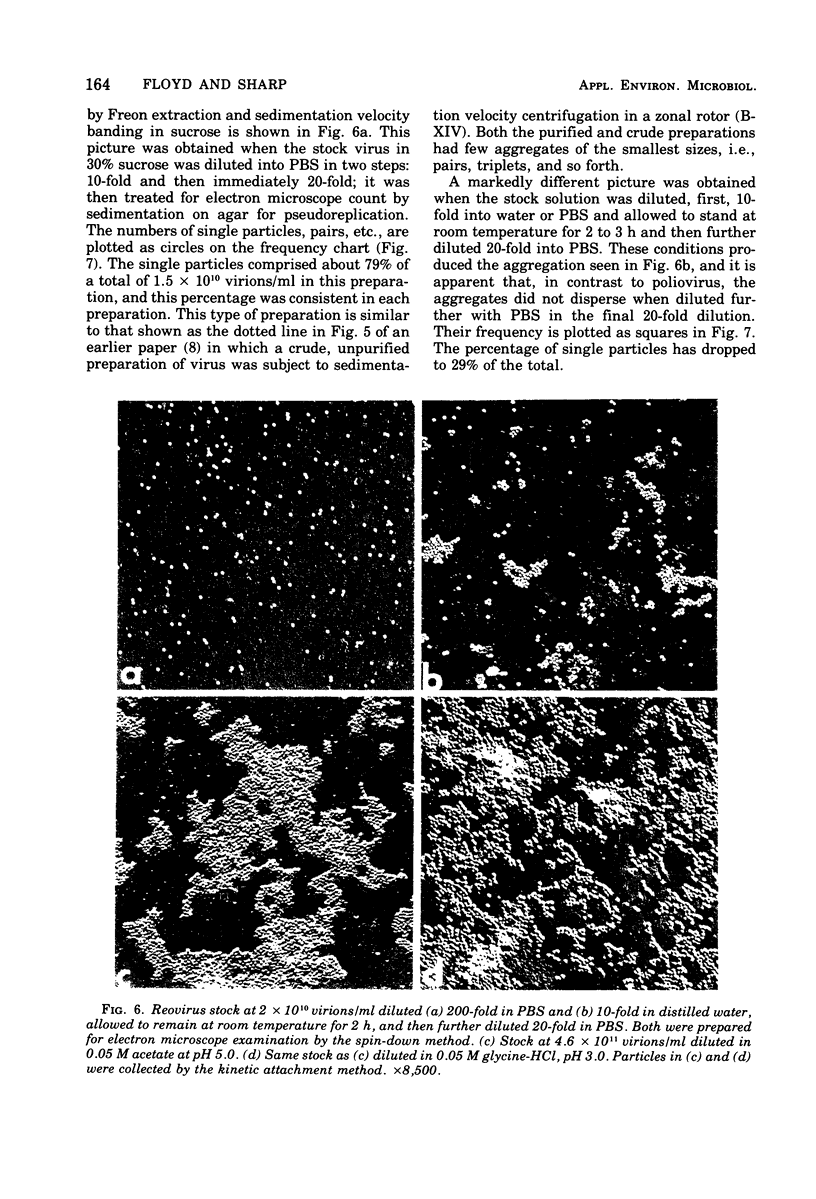
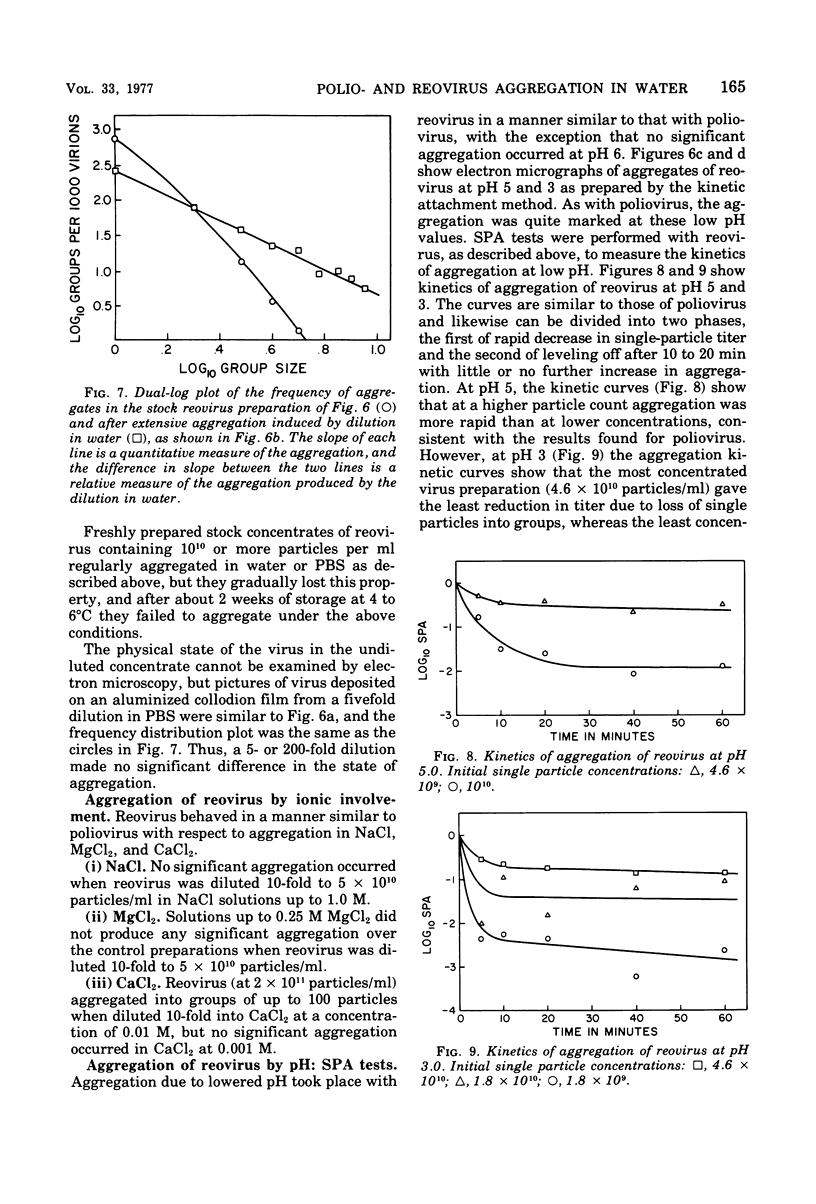
Poliovirus and reovirus were found to aggregate into clumps of up to several hundred particles when diluted 10-fold into distilled water from a stock preparation of minimal aggregation in 0.05 M phosphate buffer, pH 7.2, plus 22 to 30% sucrose. Reovirus was also found to aggregate when diluted into phosphate-buffered saline. The aggregation was concentration dependent and did not occur when either virus was diluted into water 100-fold or greater. The aggregation of poliovirus was reversible by further addition of saline and produced a dispersed preparation of virus. Reovirus aggregation was not reversible. Both viruses aggregated when diluted into buffers at pH 5 and 3, and poliovirus aggregated at pH 6, and this aggregation of both viruses was reversible when returned to pH 7. Aggregation did not occur at alkaline pH values. Aggregation at low pH could be caused aggregation of either virus at pH 7. Calcium ions, however, were found to aggregate both viruses at a concentration of 0.01 M.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Floyd R., Johnson J. D., Sharp D. G. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 1976 Feb;31(2):298–303. doi: 10.1128/aem.31.2.298-303.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Akin E. W., Benton W. H., Mayhew C. J., Metcalf T. G. Recovery of poliovirus from turbid estuarine water on microporous filters by the use of celite. Appl Microbiol. 1974 Mar;27(3):506–512. doi: 10.1128/am.27.3.506-512.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. B., Bodily A. S. Effect of environmental pH on adenovirus-associated virus. Proc Soc Exp Biol Med. 1975 Dec;150(3):585–590. doi: 10.3181/00379727-150-39085. [DOI] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Initial fast reaction of bromine on reovirus in turbulent flowing water. Appl Environ Microbiol. 1976 Feb;31(2):173–181. doi: 10.1128/aem.31.2.173-181.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Nature of the surviving plaque-forming unit of reovirus in water containing bromine. Appl Microbiol. 1975 Jan;29(1):94–101. doi: 10.1128/am.29.1.94-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Henderson M., Melnick J. L. Concentration of enteroviruses from large volumes of water. Appl Microbiol. 1973 Oct;26(4):529–534. doi: 10.1128/am.26.4.529-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Henderson M., Melnick J. L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972 Mar;23(3):476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of viruses on aluminum and calcium salts. Am J Epidemiol. 1967 May;85(3):459–468. doi: 10.1093/oxfordjournals.aje.a120708. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Fields J. E. Concentration and purification of viruses by adsorption to and elution from insoluble polyelectrolytes. Appl Microbiol. 1971 Apr;21(4):703–709. doi: 10.1128/am.21.4.703-709.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. C., Sharp D. G. Poliovirus aggregates and their survival in water. Appl Environ Microbiol. 1977 Jan;33(1):168–177. doi: 10.1128/aem.33.1.168-177.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]