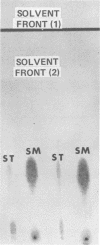
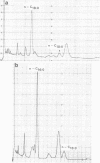
Abstract
Fatty acid fingerprints were determined gas chromatographically for Strepcococcus mutans NCTC 1082 which had been grown in batch brain heart infusion at a series of nine temperatures ranging from 29.0 to 40.0 degrees C. The major acids at all temperatures were n-palmitic and octadecenoic acids. Other acids detected at all temperatures included n-myristic, palmitoleic, n-stearic, and eicosenoic acids. An increase in temperature resulted in a decrease in the proportion of unsaturated to saturated fatty acids, indicating the importance of accurate temperature control in such gas-liquid chromatographic, chemotaxonomic studies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL K., DESCHMERTZING H., PETERSON J. I. CLASSIFICATION OF MICROORGANISMS BY ANALYSIS OF CHEMICAL COMPOSITION. I. FEASIBILITY OF UTILIZING GAS CHROMATOGRAPHY. J Bacteriol. 1963 May;85:1039–1044. doi: 10.1128/jb.85.5.1039-1044.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstein C. F., Hartman P. A. Differentiation of some enterococci by gas chromatography. J Bacteriol. 1973 Jan;113(1):38–41. doi: 10.1128/jb.113.1.38-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. B., Weaver R. E., Tatum H. W., Billingsley S. A. Differentiation between Pseudomonas testosteroni and P. acidovorans by gas chromatography. Can J Microbiol. 1972 Sep;18(9):1477–1482. doi: 10.1139/m72-226. [DOI] [PubMed] [Google Scholar]
- Drucker D. B., Green R. M., Blackmore D. K. Caries induced in rats in three weeks by a streptococcus isolated from man. J Dent Res. 1972 Sep-Oct;51(5):1510–1510. doi: 10.1177/00220345720510055201. [DOI] [PubMed] [Google Scholar]
- Drucker D. B., Griffit C. J., Melville T. H. Fatty acid fingerprints of Streptococcus mutans grown in a chemostat. Microbios. 1973;7(25):17–23. [PubMed] [Google Scholar]
- Drucker D. B., Griffith C. J., Melville T. H. The influence of vitamin and magnesium limitations on fatty-acid fingerprints of chemostat grown Streptococcus sp. SS. Microbios. 1974 Apr;10(38):183–185. [PubMed] [Google Scholar]
- Drucker D. B., Owen I. Chemotaxonomic fatty acid fingerprints of bacteria grown with, and without, aeration. Can J Microbiol. 1973 Feb;19(2):247–250. doi: 10.1139/m73-037. [DOI] [PubMed] [Google Scholar]
- Farshtchi D., McClung N. M. Effect of substrate on fatty acid production in Nocardia asteroides. Can J Microbiol. 1970 Apr;16(4):213–217. doi: 10.1139/m70-039. [DOI] [PubMed] [Google Scholar]
- Jantzen E., Bergan T., Bovre K. Gas chromatography of bacterial whole cell methanolysates; VI. Fatty acid composition of strains within Micrococcaceae;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):785–798. [PubMed] [Google Scholar]
- KATES M., ADAMS G. A., MARTIN S. M. LIPIDS OF SERRATIA MARCESCENS. Can J Biochem. 1964 Apr;42:461–479. doi: 10.1139/o64-054. [DOI] [PubMed] [Google Scholar]
- KATES M., HAGEN P. O. INFLUENCE OF TEMPERATURE ON FATTY ACID COMPOSITION OF PSYCHROPHILIC AND MESOPHILIC SERRATIA SPECIES. Can J Biochem. 1964 Apr;42:481–488. doi: 10.1139/o64-055. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J Bacteriol. 1967 Mar;93(3):894–903. doi: 10.1128/jb.93.3.894-903.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. N. Fatty acid composition of unicellular strains of blue-green algae. J Bacteriol. 1972 Feb;109(2):827–834. doi: 10.1128/jb.109.2.827-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon A., Klibansky Y., Kohn A. Detection of encephalomyocarditis virus infection in animal cells by gas liquid chromatography. Experientia. 1973 Oct 15;29(10):1305–1307. doi: 10.1007/BF01935132. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lewis V. J. Characterization of clostridia by gas chromatography. I. Differentiation of species by cellular fatty acids. Appl Microbiol. 1967 Mar;15(2):390–397. doi: 10.1128/am.15.2.390-397.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Karlson A. G., Ellefson R. D. Differentiation between Mycobacterium kansasii and Mycobacterium marinum by gas-liquid chromatographic analysis of cellular fatty acids. Appl Microbiol. 1972 Dec;24(6):1009–1010. doi: 10.1128/am.24.6.1009-1010.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]