Abstract
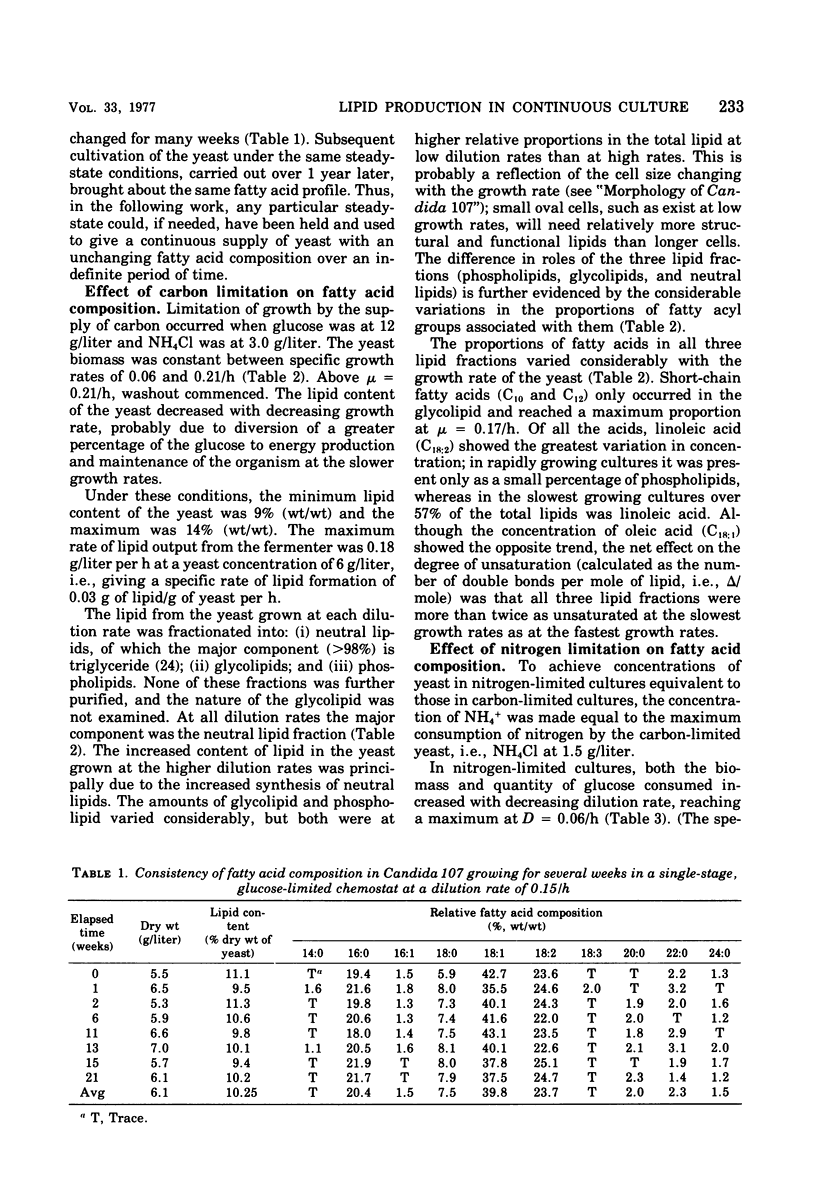
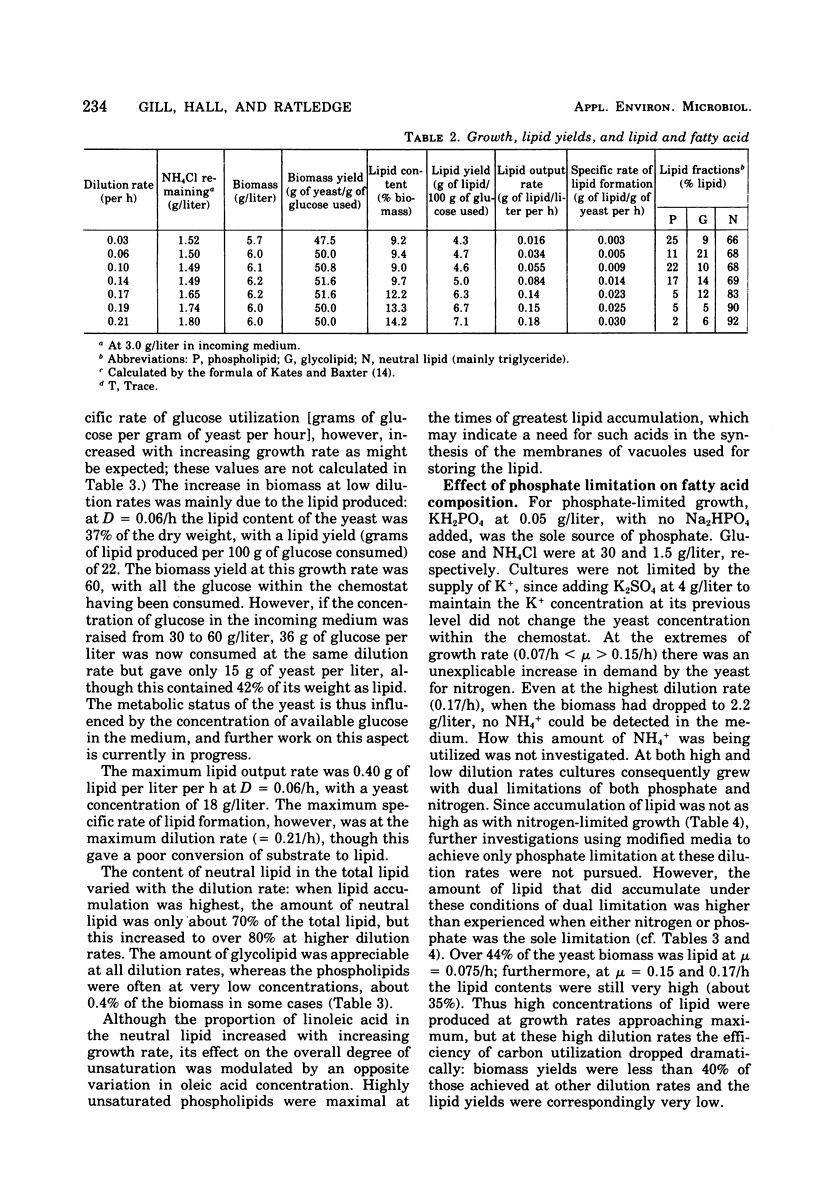
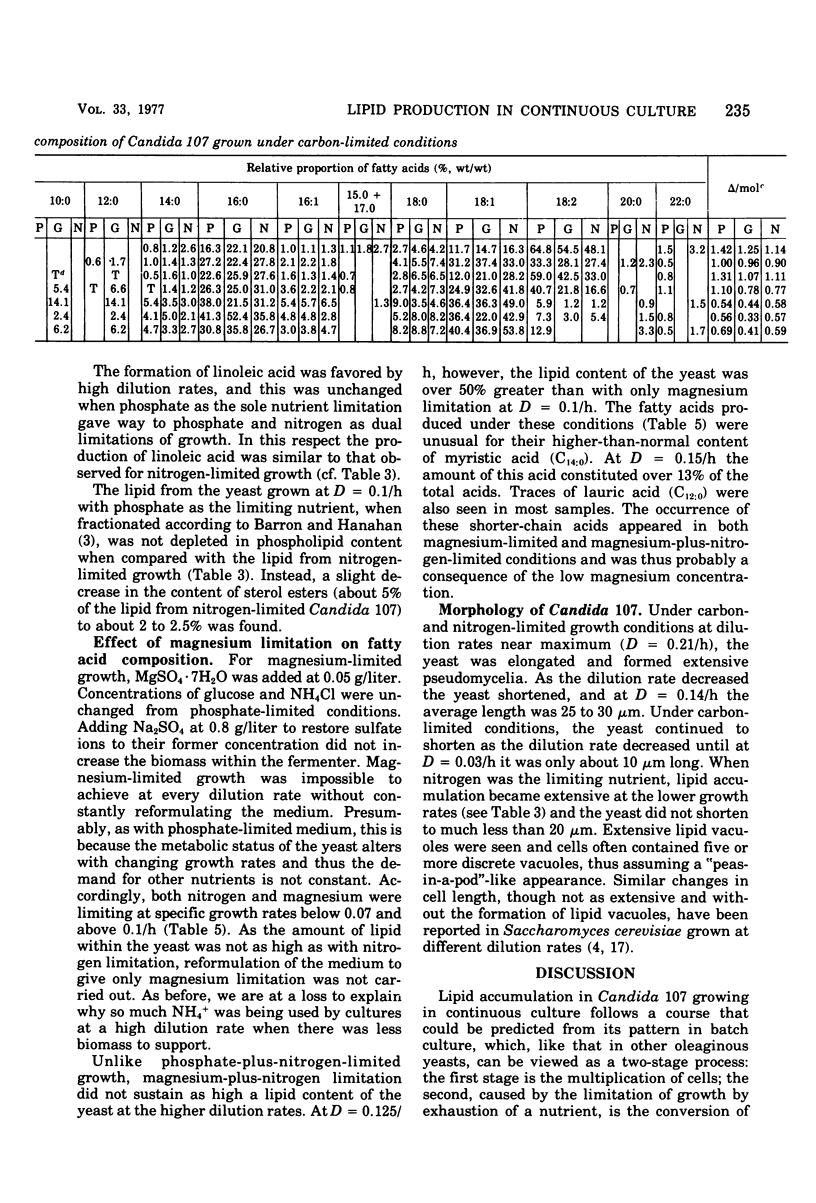
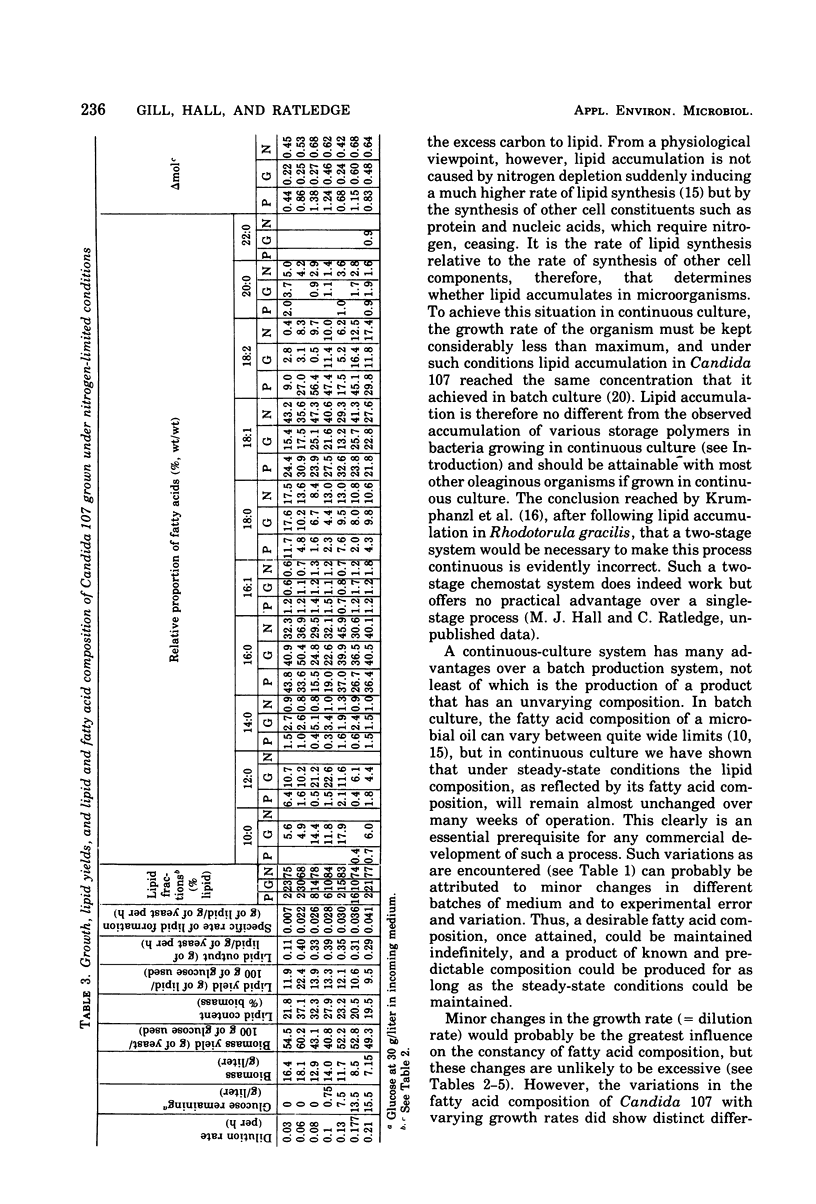
Lipid accumulation of Candida 107, grown at dilution rates from 0.03 to the maximum of 0.21/h, with carbon, nitrogen, phosphate, and magnesium limitations in a chemostat, was maximal at about 40% (wt/wt) with nitrogen-limited medium at a dilution rate of 0.06/h, giving an efficiency of substrate conversion of 22 g of lipid per g of glucose consumed. At higher dilution rates the lipid content decreased. With carbon-limited growth, the highest lipid content (14%, wt/wt) was at the maximum dilution rate. High lipid contents also occurred with phosphate + nitrogen as double limitations of growth, with the lipid content of the yeast (about 35%, wt/wt) continuing to be near maximum at dilution rates also near maximum (0.17/h), thus giving the highest specific rate of lipid formation of any growth conditions (0.59 g of lipid/g of yeast per h). However, the efficiency of substrate utilization was only 5.2 g of lipid formed per 100 g of glucose consumed. The composition of the fatty acyl residues within the lipid remained constant over many weeks if the steady-state conditions remained unchanged. With carbon-limited growth, the degree of unsaturation of the fatty acids markedly decreased as the dilution rate was increased, but with nitrogen limitation the reverse trend was seen. In all cases, linoleic and oleic acids were the principal fatty acyl residues affected, and their relative proportions always varied in opposite directions. When magnesium was a limiting nutrient, there was a considerable increase in the proportion of myristic acid produced within the lipid. Neutral lipids (predominantly triglycerides) varied from 66 to 92% of the total lipid from carbon- and nitrogen-limited growth; phospholipids (varying from 2 to 25%) were highest in nitrogen-limited growth. The fatty acyl residues within each lipid fraction showed the same variations with changing growth rates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRON E. J., HANAHAN D. J. Observations on the silicic acid chromatography of the neutral lipides of rat liver, beef liver, and yeast. J Biol Chem. 1958 Mar;231(1):493–503. [PubMed] [Google Scholar]
- Babij T., Moss F. J., Ralph B. J. Effects of oxygen and glucose levels on lipid composition of yeast Candida utilis grown in continuous culture. Biotechnol Bioeng. 1969 Jul;11(4):593–603. doi: 10.1002/bit.260110407. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Hough J. S. Protein-disulphide reductase activity in yeast. Nature. 1966 Jul 9;211(5045):201–201. doi: 10.1038/211201a0. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Rose A. H. Fatty-acid composition of Candida utilis as affected by growth temperature and dissolved-oxygen tension. J Bacteriol. 1969 Aug;99(2):371–378. doi: 10.1128/jb.99.2.371-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Dawson P. S., Craig B. M. Lipids of Candida utilis: changes with growth. Can J Microbiol. 1966 Aug;12(4):775–785. doi: 10.1139/m66-105. [DOI] [PubMed] [Google Scholar]
- Duncan B. Nutrition and fat production in submerged cultures of a strain of Penicillium lilacinum. Mycologia. 1973 Jan-Feb;65(1):211–214. [PubMed] [Google Scholar]
- Enebo L., Iwamoto H. Effects of cultivation temperature on fatty acid composition in Rhodotorula gracilis. Acta Chem Scand. 1966;20(2):439–443. doi: 10.3891/acta.chem.scand.20-0439. [DOI] [PubMed] [Google Scholar]
- Hunter K., Rose A. H. Lipid composition of Saccharomyces cerevisiae as influenced by growth temperature. Biochim Biophys Acta. 1972 Apr 18;260(4):639–653. doi: 10.1016/0005-2760(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Johnson B., Nelson S. J., Brown C. M. Influence of glucose concentration on the physiology and lipid composition of some yeasts. Antonie Van Leeuwenhoek. 1972;38(2):129–136. doi: 10.1007/BF02328084. [DOI] [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- Kessell R. H. Fatty acids of Rhodotorula gracilis: fat production in submerged culture and the particular effect of pH value. J Appl Bacteriol. 1968 Jun;31(2):220–231. doi: 10.1111/j.1365-2672.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Krumphanzl V., Grégr V., Pelechová J., Uher J. Biomass and fat production in Rhodotorula gracilis. Biotechnol Bioeng Symp. 1973;0(4-1):245–256. [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem J. 1967 Oct;105(1):189–203. doi: 10.1042/bj1050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Ratledge C., Saxton R. K. Quantitative extraction of lipid and fatty acids from a Candida species. Anal Biochem. 1968 Nov;26(2):288–294. doi: 10.1016/0003-2697(68)90339-4. [DOI] [PubMed] [Google Scholar]
- Rattray J. B., Schibeci A., Kidby D. K. Lipids of yeasts. Bacteriol Rev. 1975 Sep;39(3):197–231. doi: 10.1128/br.39.3.197-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth D. A., Ratledge C. Activities of regulatory enzymes in alkane-utilizing and lipid-accumulating yeasts and moulds. J Gen Microbiol. 1975 Sep;90(1):183–186. doi: 10.1099/00221287-90-1-183. [DOI] [PubMed] [Google Scholar]
- Whitworth D. A., Ratledge C. An analysis of intermediary metabolism and its control in a fat-synthesizing yeast (Candida 107) growing on glucose or alkanes. J Gen Microbiol. 1975 Jun;88(2):275–288. doi: 10.1099/00221287-88-2-275. [DOI] [PubMed] [Google Scholar]



