Abstract
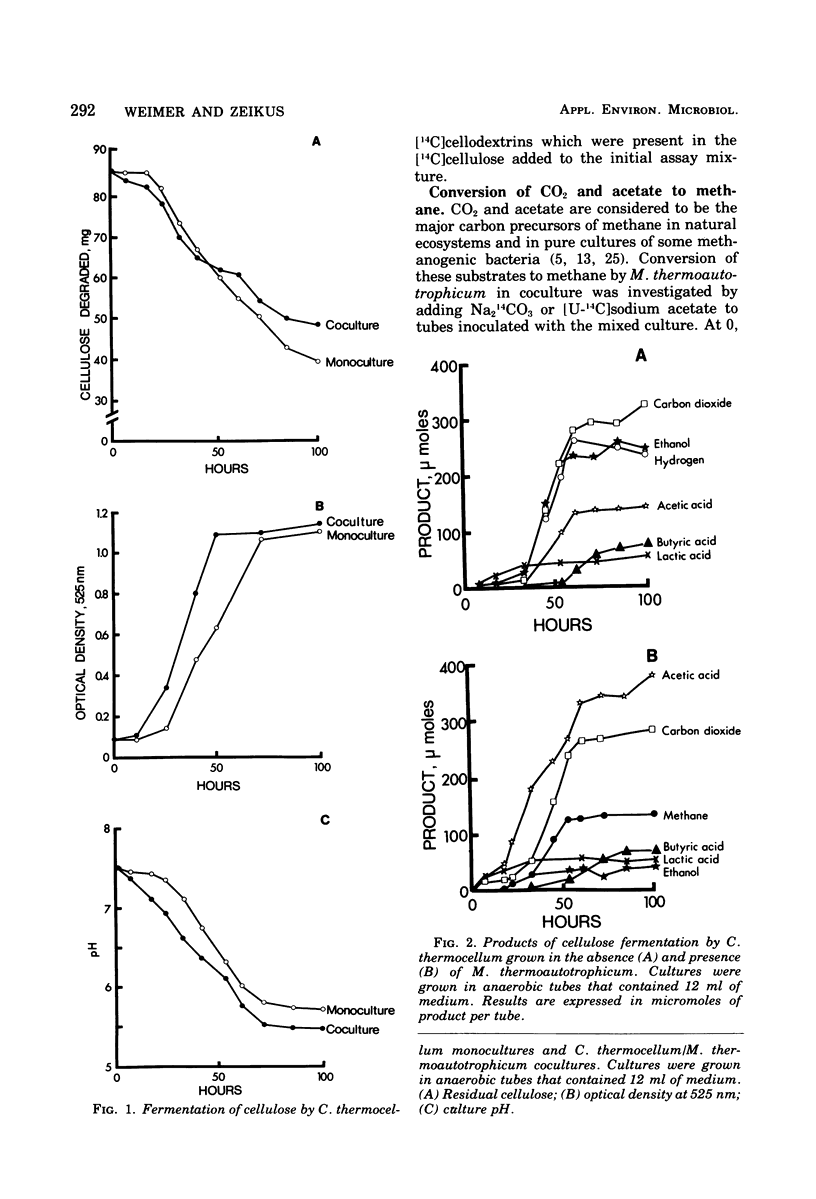
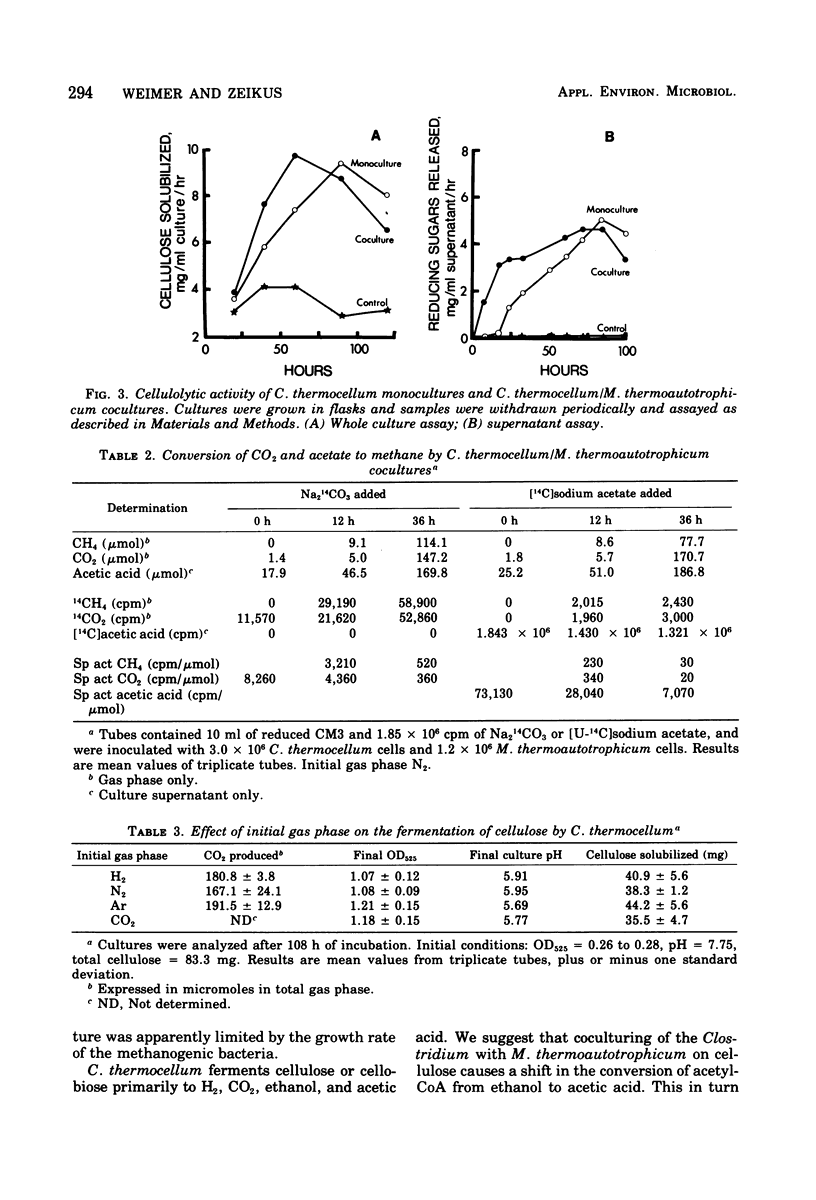
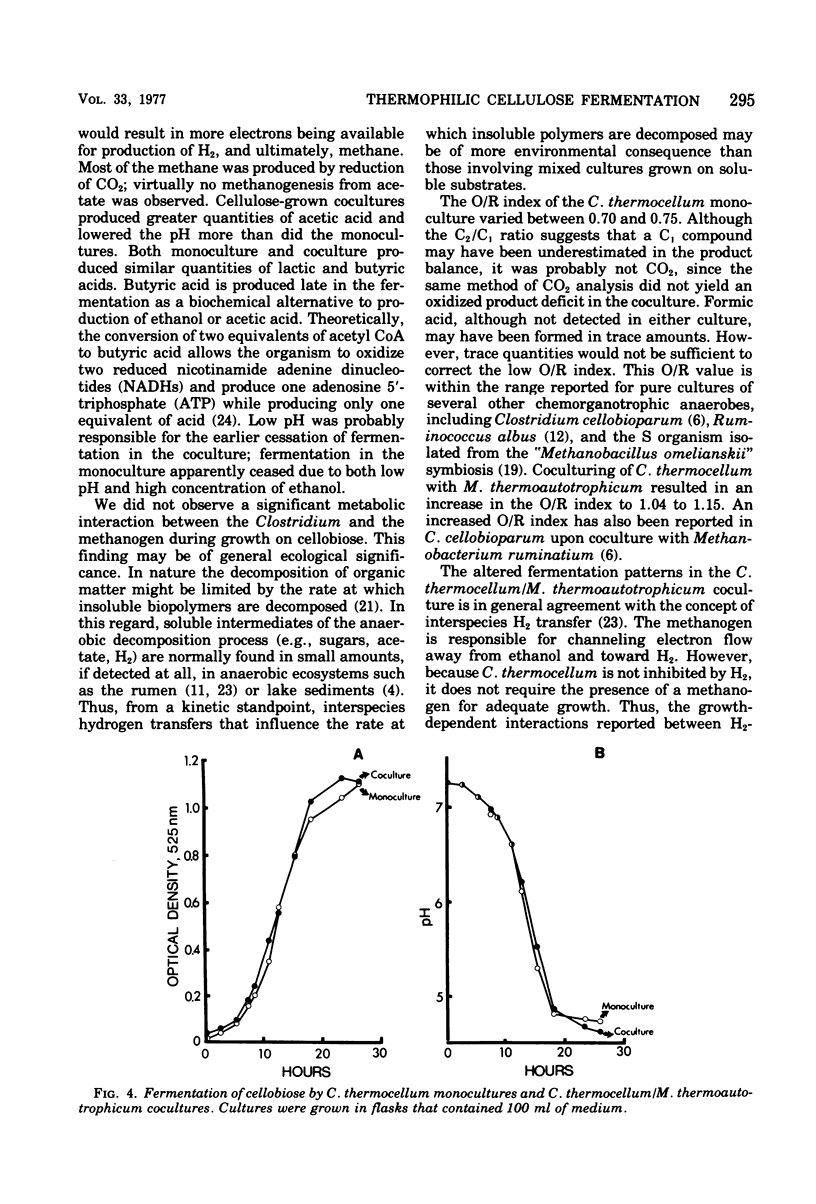
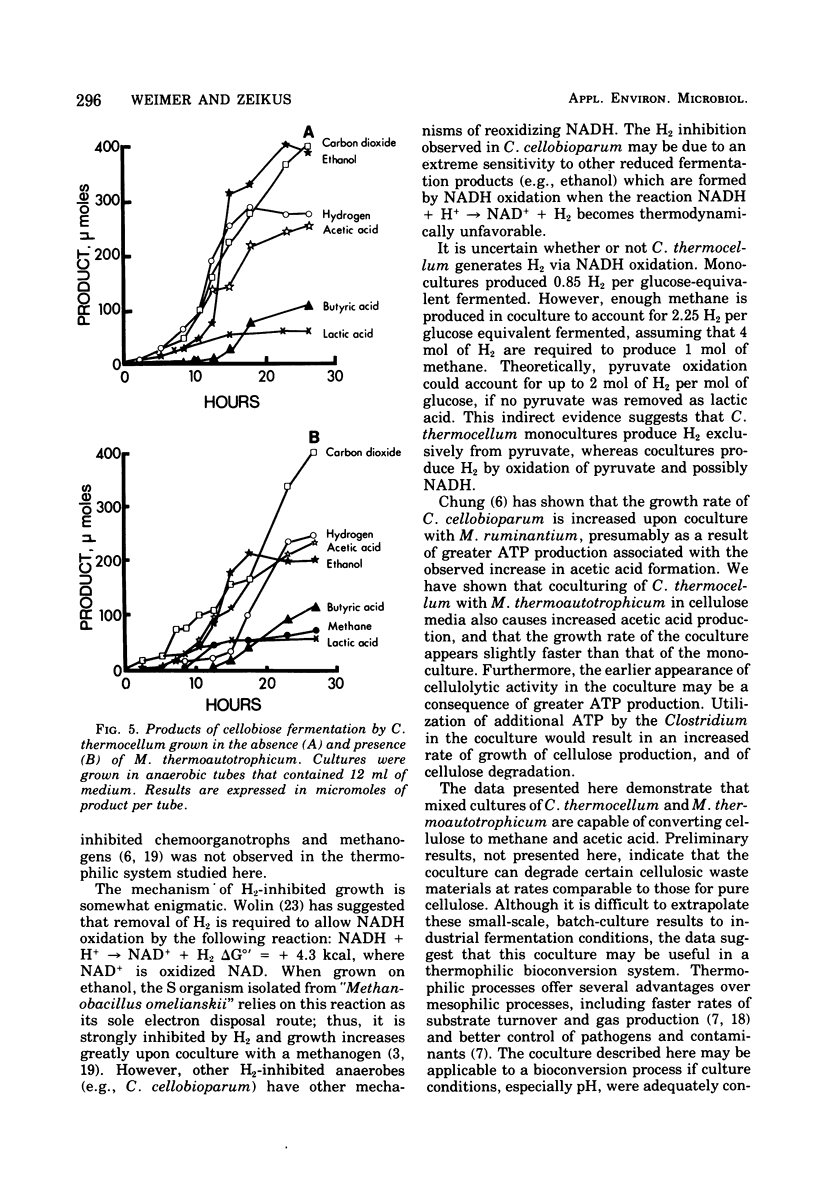
The fermentation of cellulose and cellobiose by Clostridium thermocellum monocultures and C. thermocellum/Methanobacterium thermoautotrophicum cocultures was studied. All cultures were grown under anaerobic conditions in batch culture at 60 degrees C. When grown on cellulose, the coculture exhibited a shorter lag before initiation and growth and celluloysis than did the monoculture. Cellulase activity appeared earlier in the coculture than in the monoculture; however, after growth had ceased, cellulase activity was greater in the monoculture. Monocultures produced primarily ethanol, acetic acid, H2 and CO2. Cocultures produced more H2 and acetic acid and less ethanol than did the monoculture. In the coculture, conversion of H2 to methane was usually complete, and most of the methane produced was derived from CO2 reduction rather than from acetate conversion. Agents of fermentation stoppage were found to be low pH and high concentrations of ethanol in the monoculture and low pH in the coculture. Fermentation of cellobiose was more rapid than that of cellulose. In cellobiose medium, the methanogen caused only slight changes in the fermentation balance of the Clostridium, and free H2 was produced.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. II. Inhibition experiments. Antonie Van Leeuwenhoek. 1974;40(2):297–306. doi: 10.1007/BF00394388. [DOI] [PubMed] [Google Scholar]
- Chung K. T. Inhibitory effects of H2 on growth of Clostridium cellobioparum. Appl Environ Microbiol. 1976 Mar;31(3):342–348. doi: 10.1128/aem.31.3.342-348.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney C. L. Thermophilic anaerobic digestion of solid waste for fuel gas production. Biotechnol Bioeng. 1975 Aug;17(8):1119–1135. doi: 10.1002/bit.260170804. [DOI] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. Improved culture flask for obligate anaerobes. Appl Microbiol. 1975 May;29(5):710–711. doi: 10.1128/am.29.5.710-711.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K. J., Zabransky R. J. Modification of the gas-liquid chromatography procedure and evaluation of a new column packing material for the identification of anaerobic bacteria. J Clin Microbiol. 1975 Jul;2(1):1–7. doi: 10.1128/jcm.2.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E. Hydrogen as an intermediate in the rumen fermentation. Arch Mikrobiol. 1967;59(1):158–164. doi: 10.1007/BF00406327. [DOI] [PubMed] [Google Scholar]
- Iannotti E. L., Kafkewitz D., Wolin M. J., Bryant M. P. Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H 2 . J Bacteriol. 1973 Jun;114(3):1231–1240. doi: 10.1128/jb.114.3.1231-1240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. H., Blackburn T. H. Cellulase production by a thermophilic clostridium species. Appl Microbiol. 1975 Sep;30(3):346–353. doi: 10.1128/am.30.3.346-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Characteristics of S organism isolated from Methanobacillus omelianskii. J Bacteriol. 1972 Feb;109(2):539–545. doi: 10.1128/jb.109.2.539-545.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C. C., Linehan B., Wolin M. J. H2 production by Selenomonas ruminantium in the absence and presence of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):480–483. doi: 10.1128/am.29.4.480-483.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- Wolin M. J. Metabolic interactions among intestinal microorganisms. Am J Clin Nutr. 1974 Nov;27(11):1320–1328. doi: 10.1093/ajcn/27.11.1320. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



