Abstract
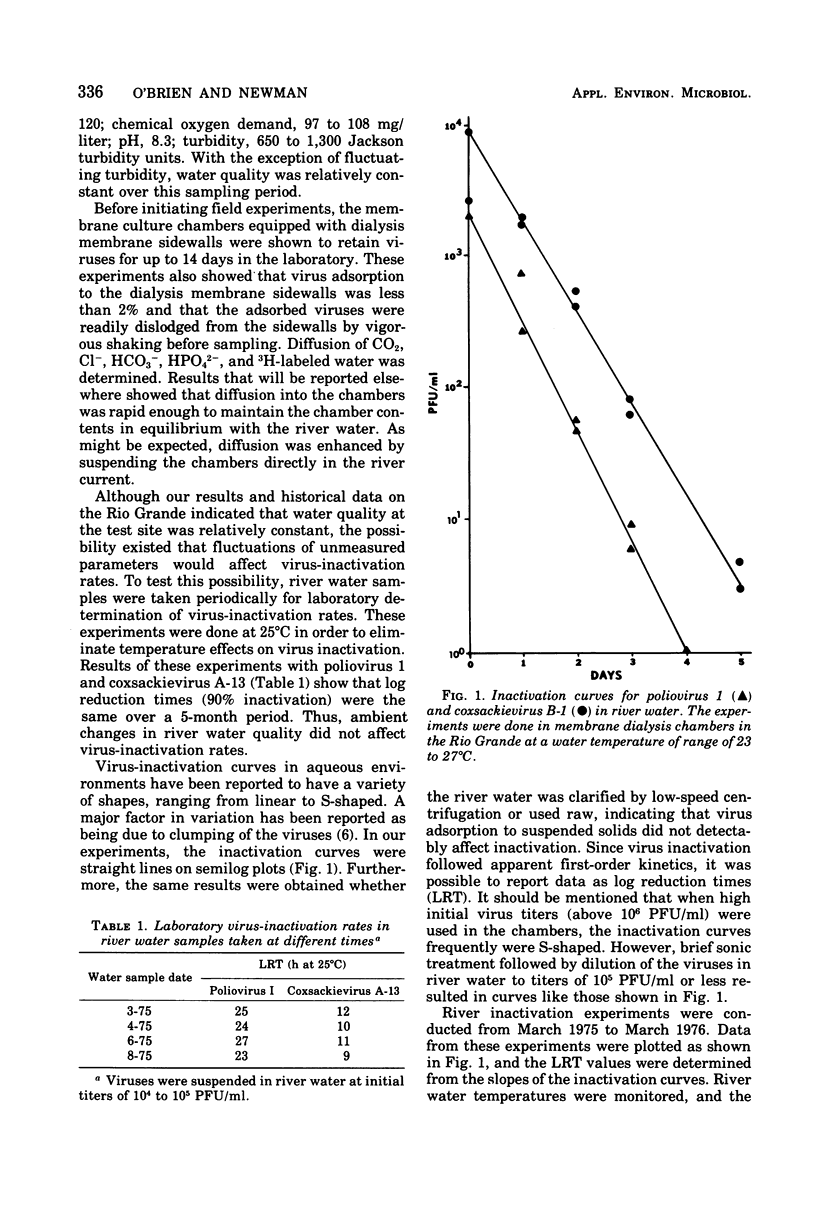
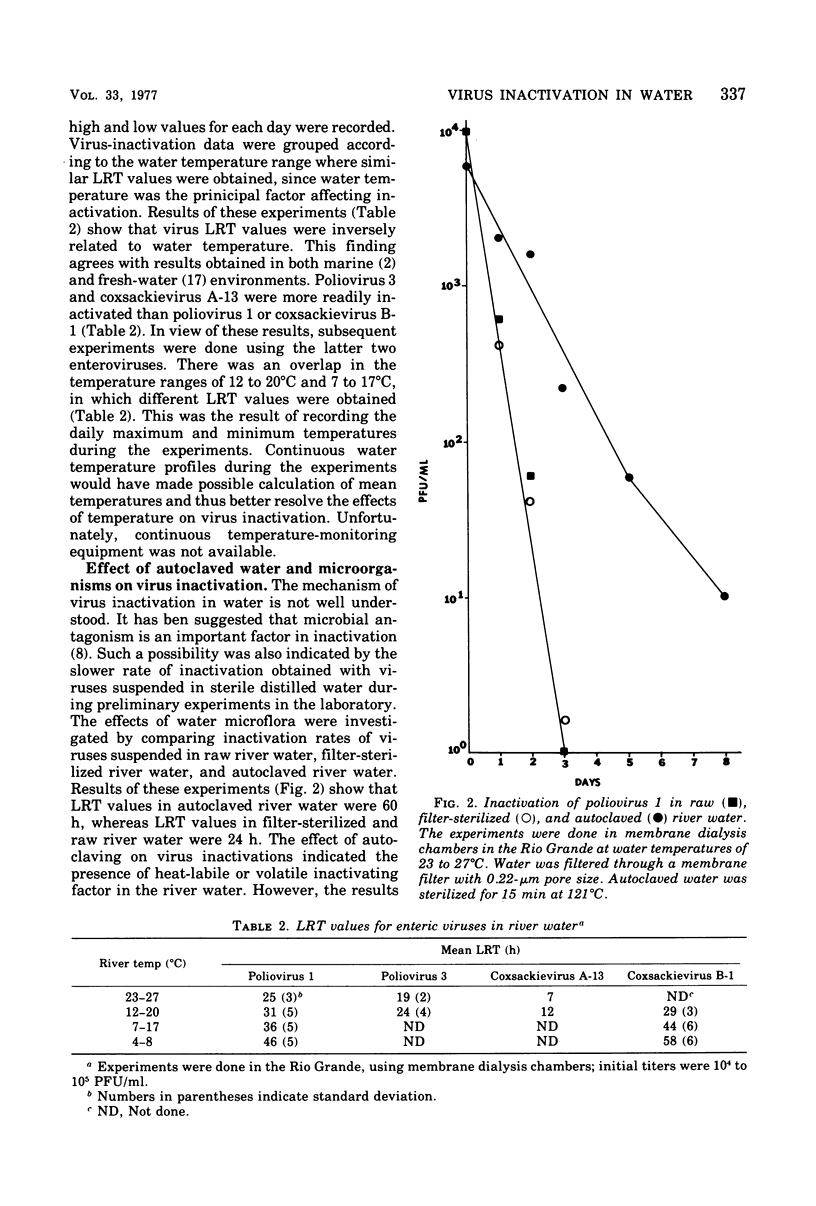
Inactivation rates of polioviruses 1 and 3 and coxsackieviruses A-13 and B-1 were determined in situ in the Rio Grande in southern New Mexico, using membrane dialysis chambers. Inactivation of the viruses was exponential, and the rates of inactivation were apparently affected principally by the water temperature. Stability of the viruses in river water differed, with poliovirus 1 and coxsackie B-1 being most stable. Typically 1-log reductions of infectivity at water temperatures ranging between 23 and 27 degrees C required 25 h for poliovirus 1, 19 h for poliovirus 3, and 7 h for coxsackie virus A-13. At water temperatures of 4 to 8 degrees C, the log reduction times for poliovirus 1 and coxsackievirus B-1 were 46 and 58 h, respectively. Results obtained with labeled poliovirus 1 and coxsackievirus B-1 and with infectious ribonucleic acid indicate that inactivation was due to damage to viral ribonucleic acid. Virus-inactivation rates were also affected by heat sterilization of river water, indicating the presence of a heat-labile or volatile inactivating factor. The inactivating factor in Rio Grande water was apparently present at a constant concentration over a 1-year period.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cords C. E., James C. G., McLaren L. C. Alteration of capsid proteins of coxsackievirus A13 by low ionic concentrations. J Virol. 1975 Feb;15(2):244–252. doi: 10.1128/jvi.15.2.244-252.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Kostenbader K. D., Jr, CLIVER D. O. Persistence of enteroviruses in lake water. Appl Microbiol. 1974 Nov;28(5):895–896. doi: 10.1128/am.28.5.895-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simková A., Wallnerová Z. Survival of small amounts of Coxsackie A4 virus in Danube river water under laboratory conditions. Acta Virol. 1973 Nov;17(6):505–506. [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Inactivation of poliovirus in digested sludge. Appl Environ Microbiol. 1976 Jun;31(6):921–930. doi: 10.1128/aem.31.6.921-930.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


