Abstract
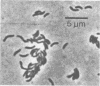
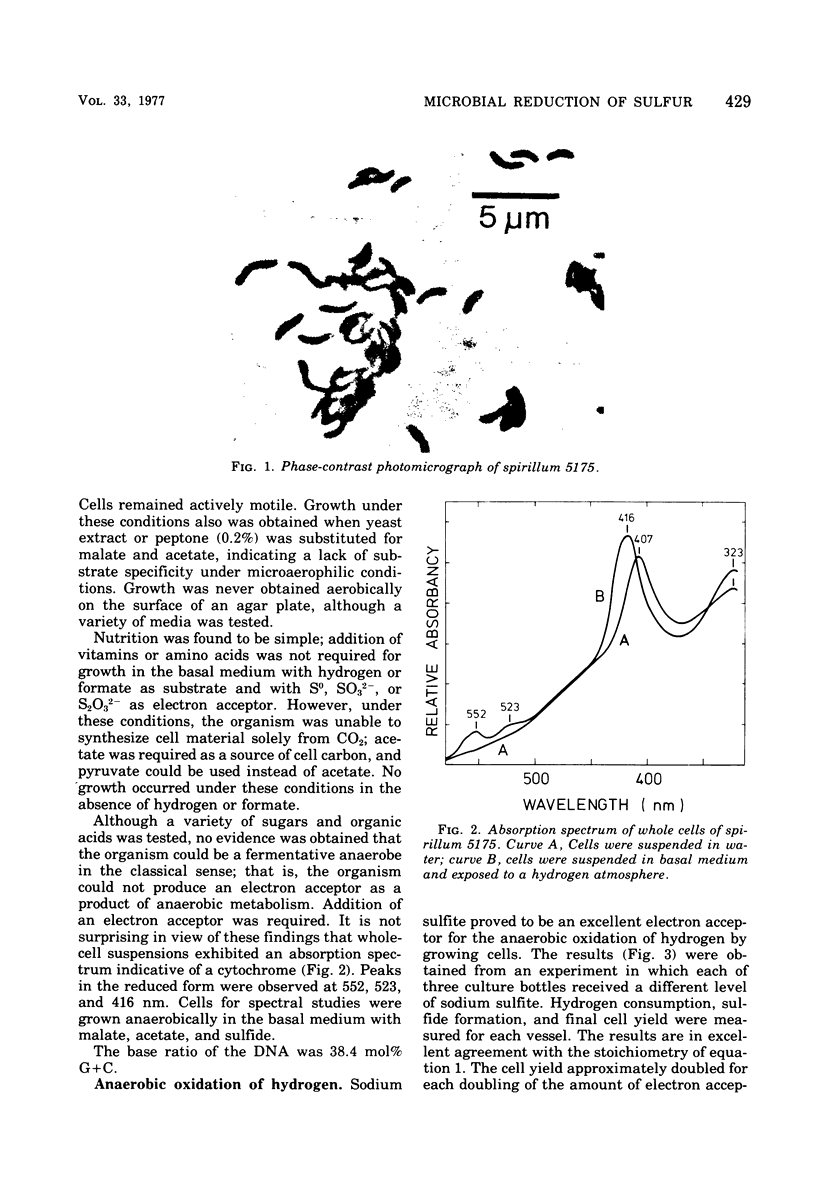
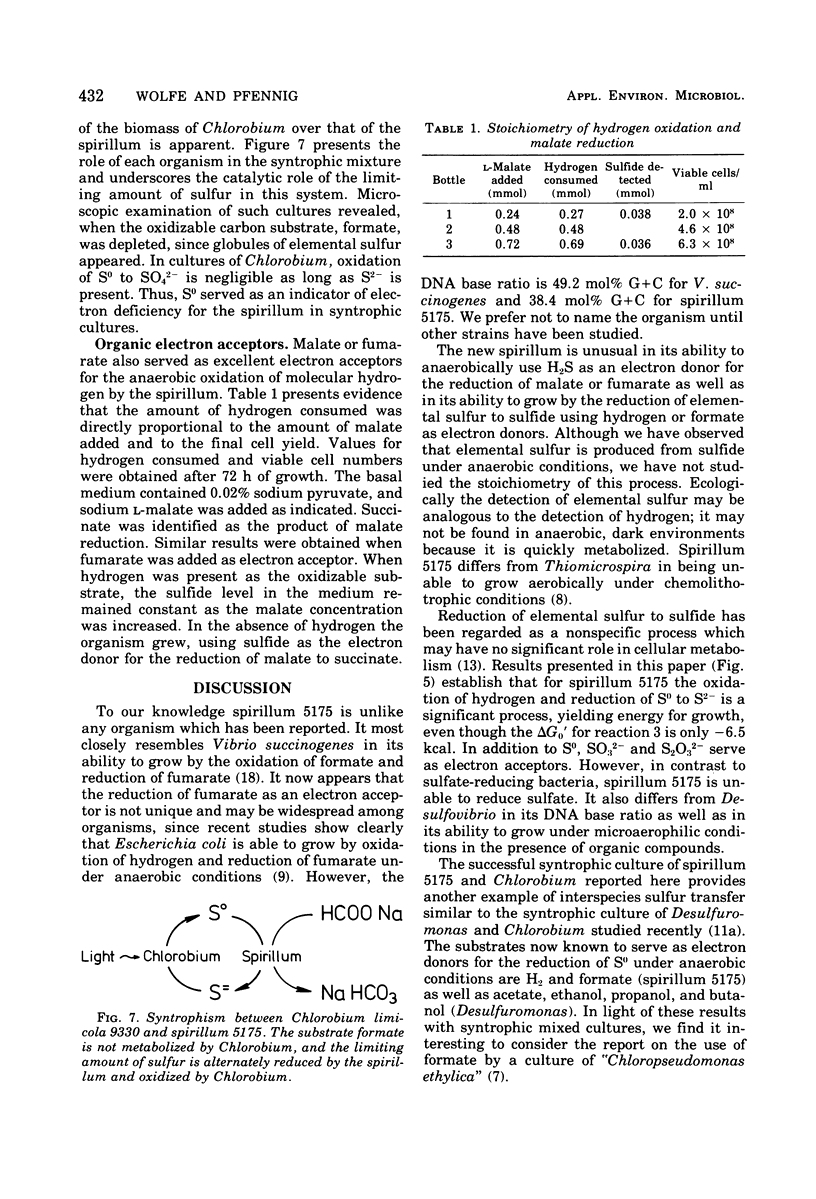
A small spirillum, designated 5175, was isolated from an anaerobic enrichment culture for Desulfuromonas in which the major medium constituents were acetate and elemental sulfur. The organisms grew only under anaerobic or microaerophilic conditions. Elemental sulfur was formed anaerobically in a malate-sulfide medium, and cell densities of 10(8) cells/ml were obtained. Hydrogen and formate were actively oxidized as substrates for growth under anaerobic conditions; S0, S032-, or S2O32-, but not SO42-, served as electron acceptors and were stoichiometrically reduced to sulfide. Malate or fumarate likewise served as electron acceptors and were reduced to succinate. Nutritional requirements were simple, no vitamins or amino acids being required. For growth in inorganic media when carbon dioxide was the only carbon source, the addition of acetate was required as a source of cell carbon. The organism is gram negative. Cells had a diameter of 0.5 mum and a wavelength of 5.0 mum. Cell suspensions exhibited an absorption spectrum indicative of a cytochrome with peaks in the reduced form at 552, 523, and 416 nm. Well growing syntrophic cultures with Chlorobium were established with formate as the substrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. R., LeGall L., Peck H. D. Evidence for the periplasmic location of hydrogenase in Desulfovibrio gigas. J Bacteriol. 1974 Nov;120(2):994–997. doi: 10.1128/jb.120.2.994-997.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Decker K., Jungermann K., Thauer R. K. Energy production in anaerobic organisms. Angew Chem Int Ed Engl. 1970 Feb;9(2):138–158. doi: 10.1002/anie.197001381. [DOI] [PubMed] [Google Scholar]
- Hungate R. E. Hydrogen as an intermediate in the rumen fermentation. Arch Mikrobiol. 1967;59(1):158–164. doi: 10.1007/BF00406327. [DOI] [PubMed] [Google Scholar]
- Kuenen J. G., Veldkamp H. Thiomicrospira pelophila, gen. n., sp. n., a new obligately chemolithotrophic colourless sulfur bacterium. Antonie Van Leeuwenhoek. 1972;38(3):241–256. doi: 10.1007/BF02328096. [DOI] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]