Abstract
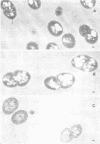
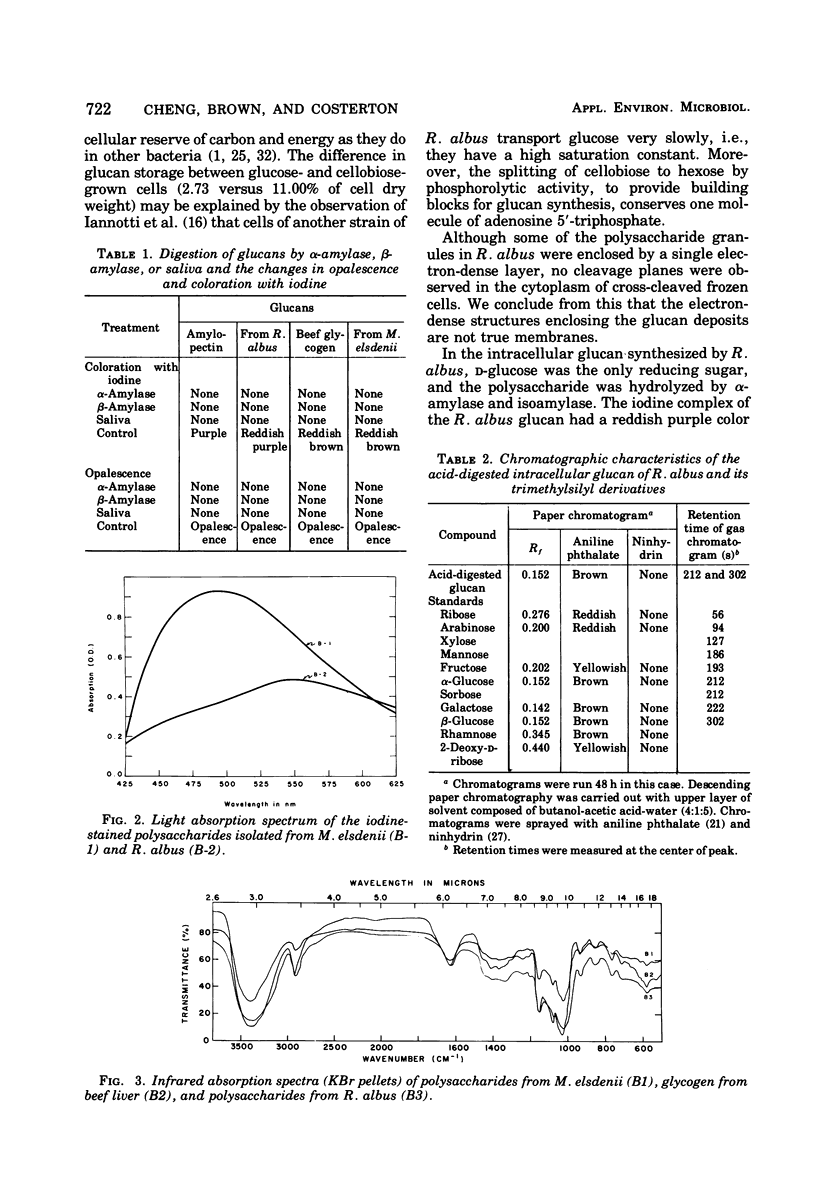
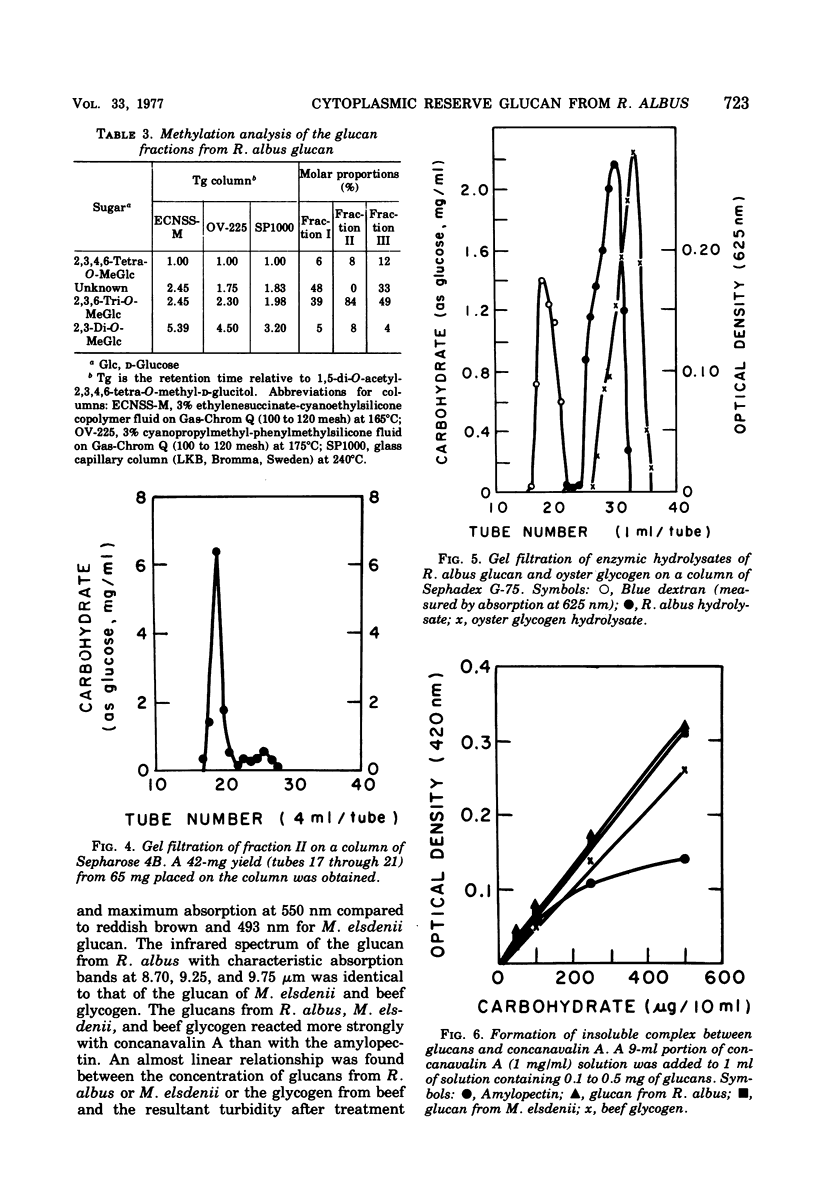
Ruminococcus albus, an anaerobic bacterium that digests cellulose in the rumen of cattle, produces intracellular polysaccharide granules varying from 0.05 to 0.31 μm in diameter when grown in batch culture. This polysaccharide material was purified and found to contain d-glucose as the only reducing sugar. The polyglucose polymer was slightly opalescent in aqueous solution and formed a strong reddish purple iodine complex with a maximum absorbance at 550 nm. Its infrared spectrum had characteristic absorption bands at 8.70, 9.25, and 9.75 μm and was identical with that of the amylopectin-glycogen type of Megasphaera elsdenii and that of the glycogen of enteric bacteria and beef liver. It reacted strongly with concanavalin A. Methylation analysis showed that the glucan contained 2,3,4,6-tetra-O-MeG-2,3,6-Tri-O-MeG-2,3-Di-O-MeG in a ratio of 8:84:8. Characterization of the products obtained by treatment with isoamylase indicates that the glucan of R. albus is of the glycogen type.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylen C. W., Pate J. L. Fine structure of Arthrobacter crystallopoietes during long-term starvation of rod and spherical stage cells. Can J Microbiol. 1973 Jan;19(1):1–5. doi: 10.1139/m73-001. [DOI] [PubMed] [Google Scholar]
- Brown R. G., Lindberg B., Cheng K. J. Characterization of a reserve glucan from Megasphaera elsdenii. Can J Microbiol. 1975 Oct;21(10):1657–1659. doi: 10.1139/m75-242. [DOI] [PubMed] [Google Scholar]
- Brown R. G., Lindberg B., Laishley E. J. Characterization of two reserve glucans from Clostridium pasteurianum. Can J Microbiol. 1975 Jul;21(7):1136–1138. doi: 10.1139/m75-168. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Hironaka R., Roberts D. W., Costerton J. W. Cytoplasmic glycogen inclusions in cells of anaerobic gram-negative rumen bacteria. Can J Microbiol. 1973 Dec;19(12):1501–1506. doi: 10.1139/m73-244. [DOI] [PubMed] [Google Scholar]
- GUTIERREZ J., DAVIS R. E., LINDAHL I. L., WARWICK E. J. Bacterial changes in the rumen during the onset of feed-lot bloat of cattle and characteristics of Peptostreptococcus elsdenii n. sp. Appl Microbiol. 1959 Jan;7(1):16–22. doi: 10.1128/am.7.1.16-22.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Marshall J. J., Mercier C., Smith E. E., Whelan W. J. A revision of the Meyer-Bernfeld model of glycogen and amylopectin. FEBS Lett. 1970 Dec 28;12(2):101–104. doi: 10.1016/0014-5793(70)80573-7. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z., Marshall J. J., Smith E. E., Whelan W. J. A glycogen-debranching enzyme from Cytophaga. FEBS Lett. 1970 Dec 28;12(2):96–100. doi: 10.1016/0014-5793(70)80572-5. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti E. L., Kafkewitz D., Wolin M. J., Bryant M. P. Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H 2 . J Bacteriol. 1973 Jun;114(3):1231–1240. doi: 10.1128/jb.114.3.1231-1240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L., Spearman T. N., Hamilton I. R. Isolation and characterization of glycogen from Streptococcus salivarius. Can J Biochem. 1972 Apr;50(4):440–442. doi: 10.1139/o72-059. [DOI] [PubMed] [Google Scholar]
- LEVINE S., STEVENSON H. J., TABOR E. C., BORDNER R. H., CHAMBERS L. A. Glycogen of enteric bacteria. J Bacteriol. 1953 Dec;66(6):664–670. doi: 10.1128/jb.66.6.664-670.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBBONS D. W., DAWES E. A. Environmental and growth conditions affecting the endogenous metabolism of bacteria. Ann N Y Acad Sci. 1963 Jan 21;102:564–586. doi: 10.1111/j.1749-6632.1963.tb13661.x. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGAL N., CATTANEO J., SEGEL I. H. GLYCOGEN ACCUMULATION BY WILD-TYPE AND URIDINE DIPHOSPHATE GLUCOSE PYROPHOSPHORYLASE-NEGATIVE STRAINS OF ESCHERICHIA COLI. Arch Biochem Biophys. 1964 Dec;108:440–451. doi: 10.1016/0003-9861(64)90425-4. [DOI] [PubMed] [Google Scholar]
- WELCH N. L., DANIELSON W. H. The effect of different methods of precipitation of protein on the enzymatic determination of blood glucose. Am J Clin Pathol. 1962 Sep;38:251–255. doi: 10.1093/ajcp/38.3.251. [DOI] [PubMed] [Google Scholar]
- Whyte J. N., Strasdine G. A. An intracellular alpha-D-glucan from Clostridium botulinum, type E. Carbohydr Res. 1972 Dec;25(2):435–441. doi: 10.1016/s0008-6215(00)81655-9. [DOI] [PubMed] [Google Scholar]