Abstract
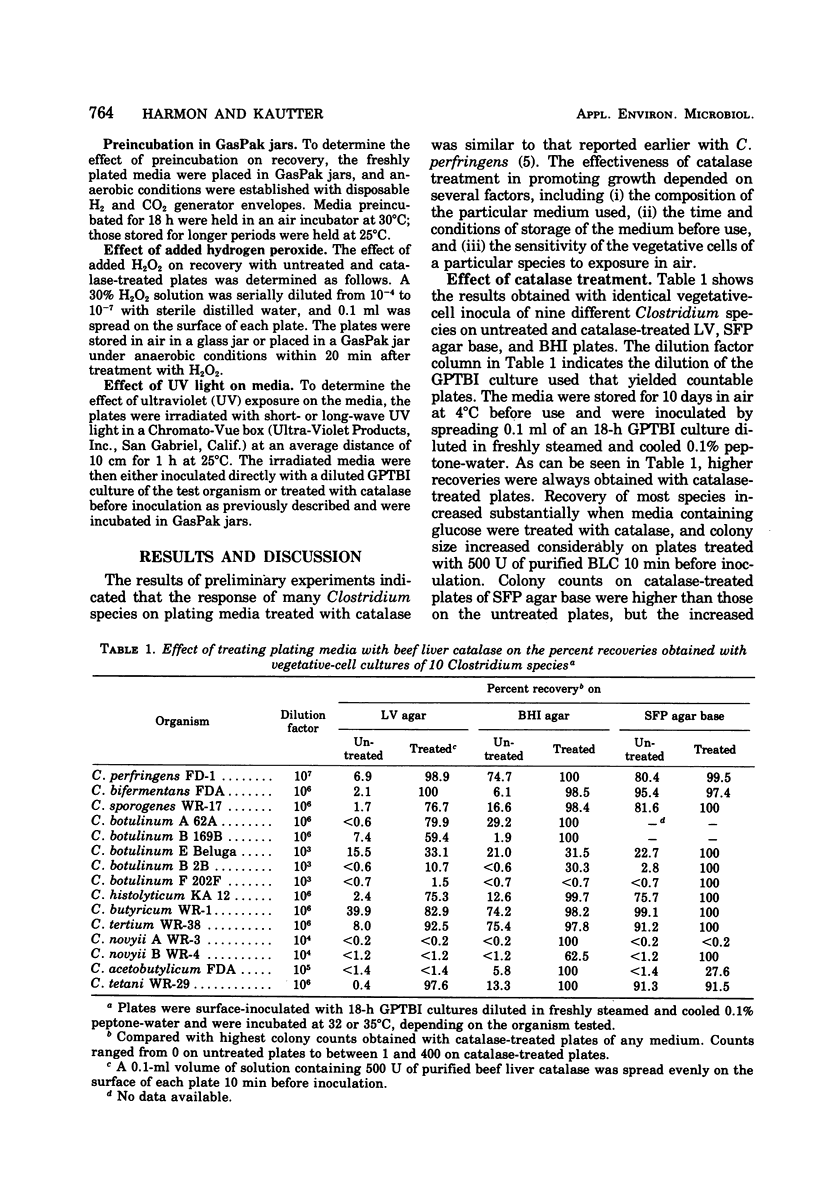
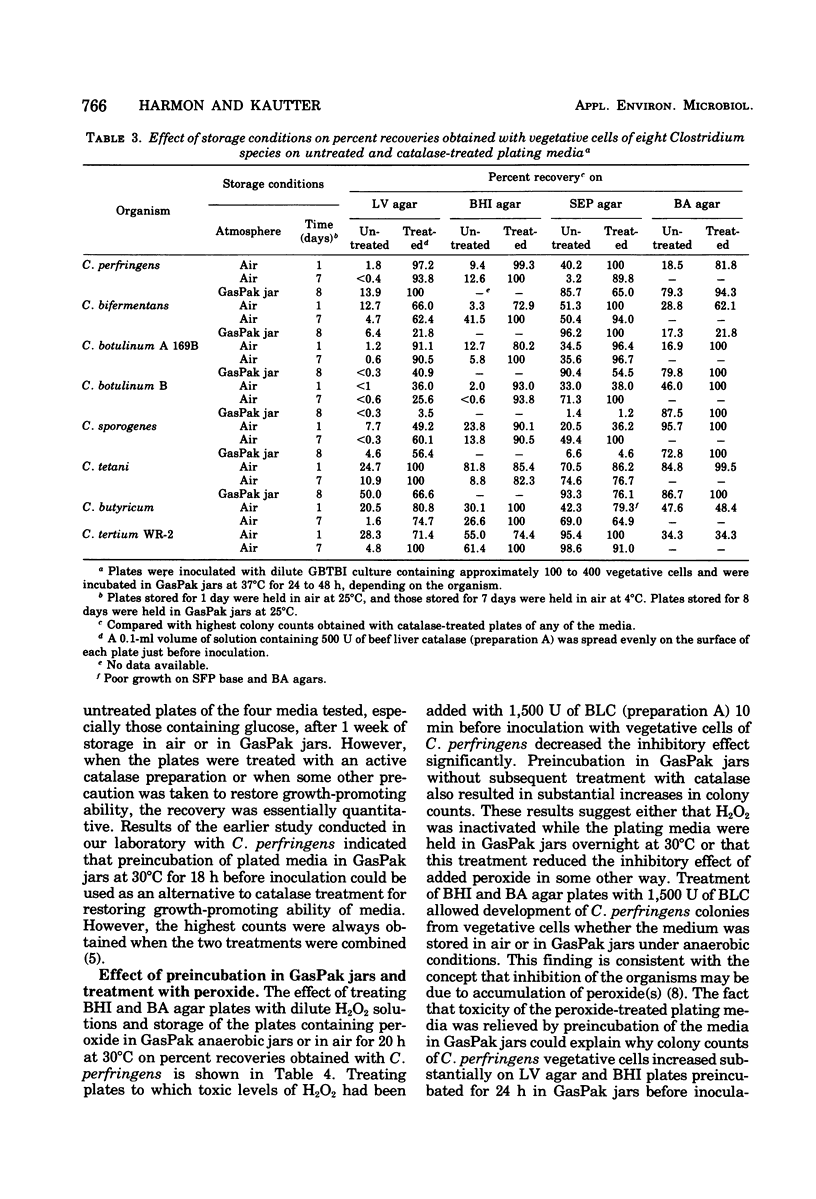
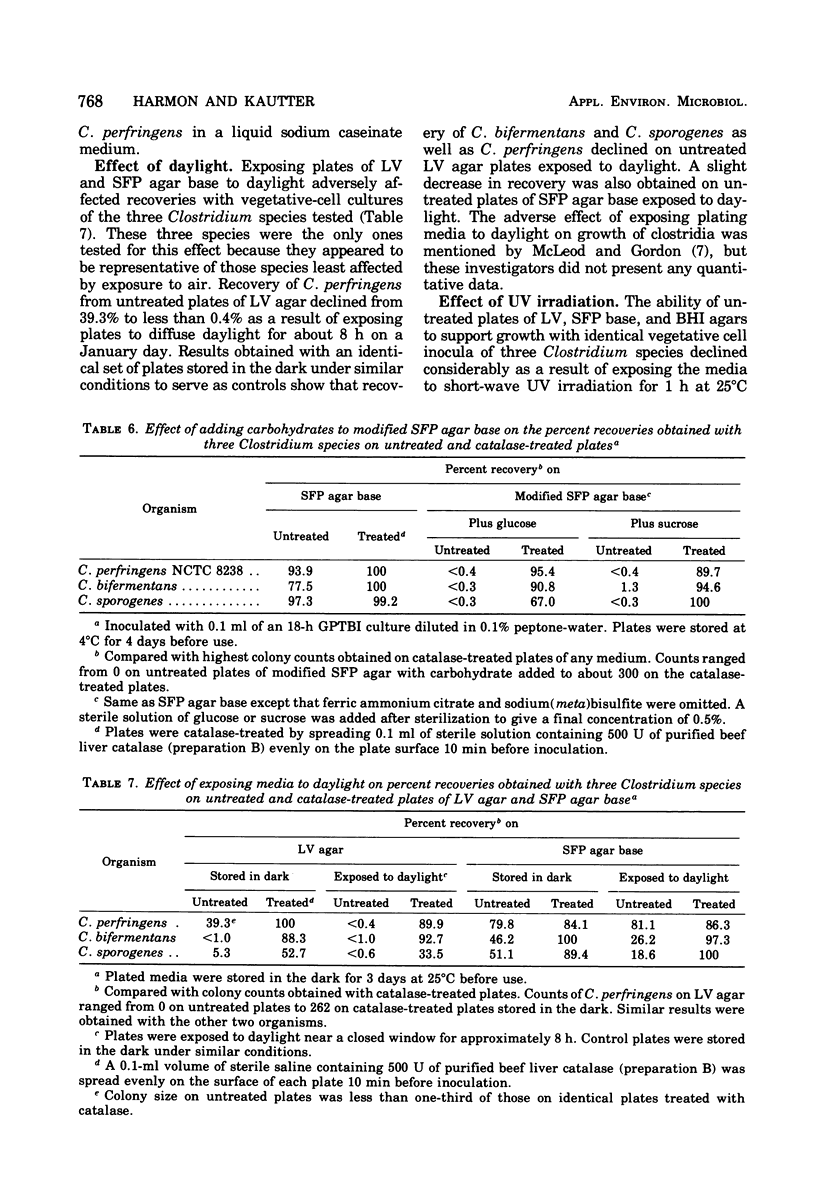
Four plating media commonly used for culturing clostridia were tested for their ability to support growth of several Clostridium species after storage of the plates for 1 to 10 days at 4 and 25°C with and without subsequent addition of catalase. Liver-veal (LV) agar and brain heart infusion (BHI) agar rapidly became incapable of supporting growth after storage without added catalase, whereas Shahidi Ferguson perfringens agar base and Brewer anaerobic agar were less affected. Plate counts of vegetative cells of nine of the less fastidious Clostridium species on untreated LV and BHI agars, stored for 3 days at 4°C, were 60 to 90% lower than counts on catalase-treated media. Counts on Shahidi Ferguson perfringens agar base were only 1 to 24% lower on untreated medium with the same species. Addition of 500 U of purified beef liver catalase to the surface of the 3-day-old agars before inoculation resulted in substantial restoration of the ability of the media to support colony formation from vegetative cells except with the most strictly anaerobic species (nonproteolytic C. botulinum types B, E, and F, and C. novyii types A and B). A similar response was obtained with spores of the less fastidious species on catalase-treated media. Our results suggest that inhibition of most Clostridium species on LV and BHI agars may be due to accumulation of peroxide during preparation, storage, and incubation of the media, and also suggest that the presence of glucose in these media is a major factor contributing to their inability to support growth. It is believed that the addition of exogenous catalase prevents the accumulation of peroxide(s), thus allowing colony formation from vegetative cells of the clostridia under what would otherwise be unsuitable cultural conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY V. C., CONALTY M. L., DENNENY J. M., WINDER F. Peroxide formation in bacteriological media. Nature. 1956 Sep 15;178(4533):596–597. doi: 10.1038/178596a0. [DOI] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Wieler D. I. Characteristics of Clostridium botulinum type F isolated from the Pacific Coast of the United States. Appl Microbiol. 1967 Nov;15(6):1316–1323. doi: 10.1128/am.15.6.1316-1323.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON J., HOLMAN R. A., McLEOD J. W. Further observations on the production of hydrogen peroxide by anaerobic bacteria. J Pathol Bacteriol. 1953 Oct;66(2):527–537. doi: 10.1002/path.1700660224. [DOI] [PubMed] [Google Scholar]
- HOLMAN R. A. The use of catalase in the growth of anaerobes. J Pathol Bacteriol. 1955 Jul;70(1):195–204. doi: 10.1002/path.1700700117. [DOI] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A. Beneficial effect of catalase treatment on growth of Clostridium perfringens. Appl Environ Microbiol. 1976 Sep;32(3):409–416. doi: 10.1128/aem.32.3.409-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATELES R. I., ZUBER B. L. THE EFFECT OF EXOGENOUS CATALASE ON THE AEROBIC GROWTH OF CLOSTRIDIA. Antonie Van Leeuwenhoek. 1963;29:249–255. doi: 10.1007/BF02046065. [DOI] [PubMed] [Google Scholar]
- PROOM H., WOIWOD A. J., BARNES J. M., ORBELL W. G. A growth-inhibitory effect on Shigella dysenteriae which occurs with some batches of nutrient agar and is associated with the production of peroxide. J Gen Microbiol. 1950 May;4(2):270–276. doi: 10.1099/00221287-4-2-270. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Torres-Anjel M. J., Riemann H. P. Improved culture techniques and sporulation medium for enterotoxin production by Clostridium perfringens type A. Taiwan Yi Xue Hui Za Zhi. 1974 Jul;73(7):404–409. [PubMed] [Google Scholar]
- Wyss O., Clark J. B., Haas F., Stone W. S. The Role of Peroxide in the Biological Effects of Irradiated Broth. J Bacteriol. 1948 Jul;56(1):51–57. [PMC free article] [PubMed] [Google Scholar]



