Abstract
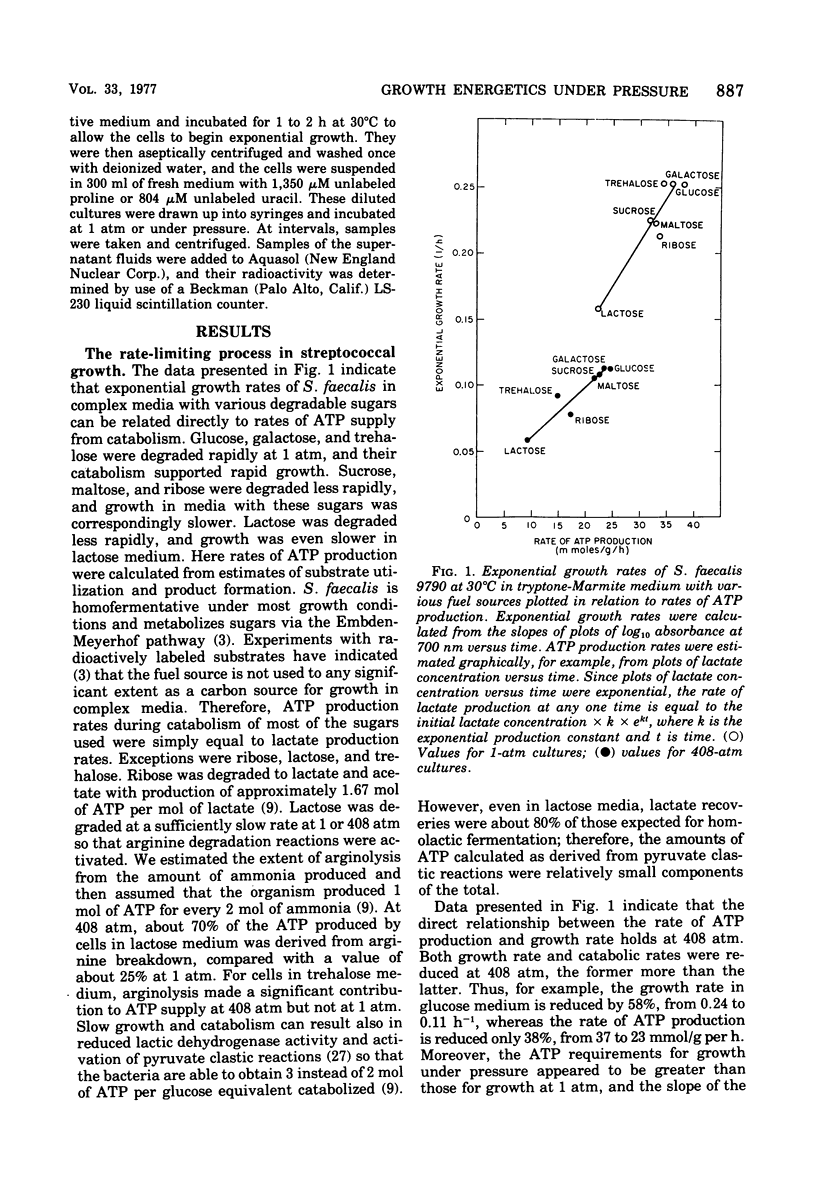
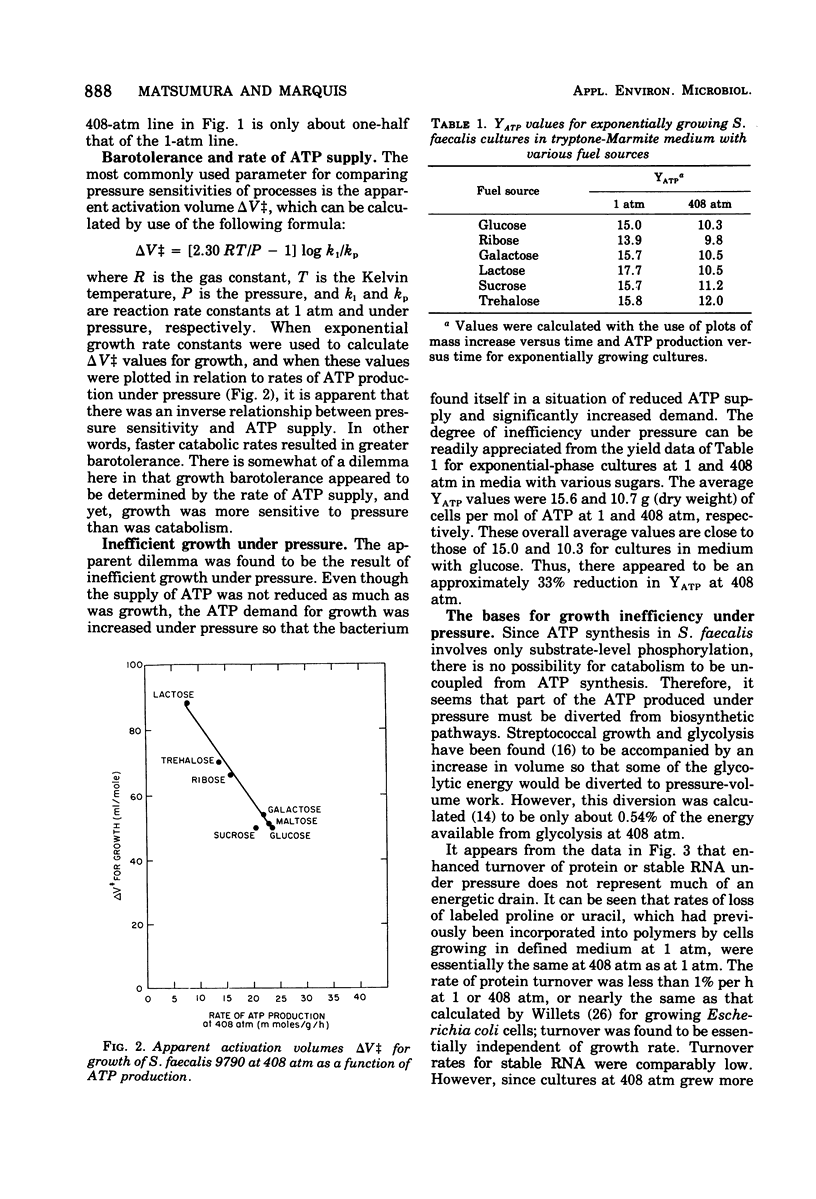
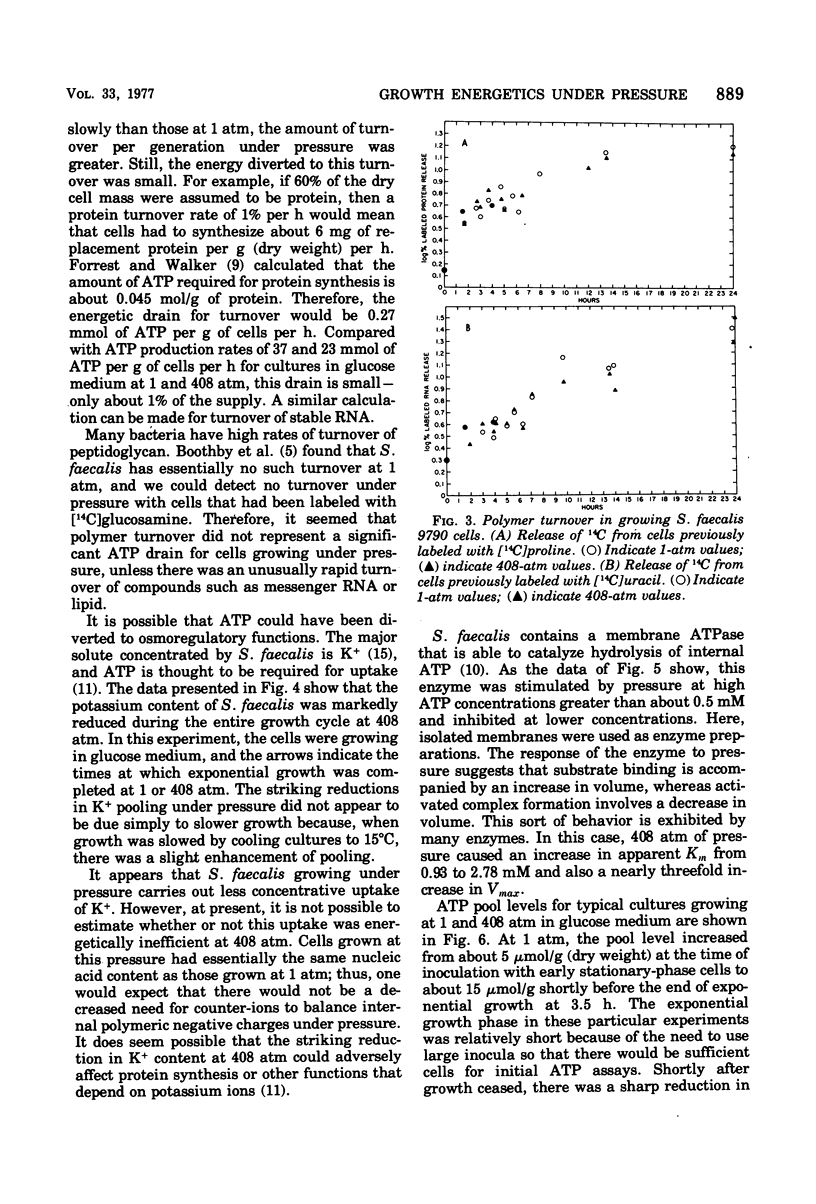
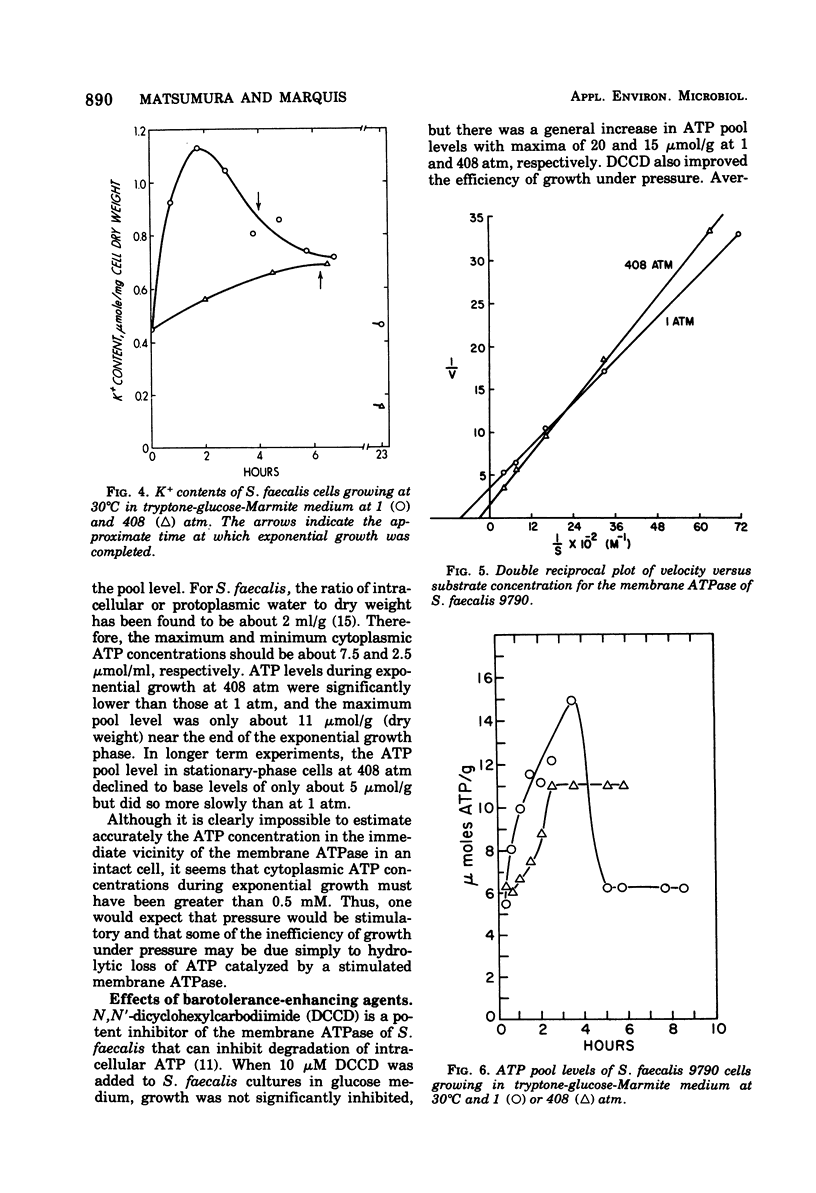
Growth of Streptococcus faecalis in complex media with various fuel sources appeared to be limited by the rate of supply of adenosine-5′ -triphosphate (ATP) at 1 atm and also under 408 atm of hydrostatic pressure. Growth under pressure was energetically inefficient, as indicated by an average cell yield for exponentially growing cultures of only 10.7 g (dry weight) per mol of ATP produced compared with a 1-atm value of 15.6. Use of ATP for pressure-volume work or for turnover of protein, peptidoglycan, or stable ribonucleic acid (RNA) did not appear to be significant causes of growth inefficiency under pressure. In addition, there did not seem to be an increased ATP requirement for ion uptake because cells growing at 408 atm had significantly lower internal K+ levels than did those growing at 1 atm. Pressure did stimulate the membrane adenosine triphosphatase (ATPase) or S. faecalis at ATP concentrations greater than 0.5 mM. Intracellular ATP levels were found to vary during the culture cycle from about 2.5 μmol/ml of cytoplasmic water for lag-phase or stationary-phase cells to maxima for exponentially growing cells of about 7.5 μmol/ml at 1 atm and 5.5 μmol/ml at 408 atm. N,N′-dicyclohexylcarbodiimide at a 10 μM concentration improved growth efficiency under pressure, as did Mg2+ or Ca2+ ions at 50 mM concentration. These agents also enhanced ATP pooling, and it seemed that at least part of the growth inefficiency under pressure was due to increased ATPase activity. In all, it appeared that S. faecalis growing under pressure has somewhat reduced ATP supply but significantly increased demand and that the inhibitory effects of pressure can be interpreted largely in terms of ATP supply and demand.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Albright L. J. The influence of hydrostatic pressure upon biochemical activities of heterotrophic bacteria. Can J Microbiol. 1975 Sep;21(9):1406–1412. doi: 10.1139/m75-210. [DOI] [PubMed] [Google Scholar]
- BHASKARAN K. Recombination of characters between mutant stocks of Vibrio cholerae, strain 162. J Gen Microbiol. 1960 Aug;23:47–54. doi: 10.1099/00221287-23-1-47. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Forrest W. W., Walker D. J. The generation and utilization of energy during growth. Adv Microb Physiol. 1971;5:213–274. doi: 10.1016/s0065-2911(08)60408-7. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Marquis R. E., Brown W. P., Fenn W. O. Pressure sensitivity of streptococcal growth in relation to catabolism. J Bacteriol. 1971 Feb;105(2):504–511. doi: 10.1128/jb.105.2.504-511.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Carstensen E. L. Electric conductivity and internal osmolality of intact bacterial cells. J Bacteriol. 1973 Mar;113(3):1198–1206. doi: 10.1128/jb.113.3.1198-1206.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Fenn W. O. Dilatometric study of streptococcal growth and metabolism. Can J Microbiol. 1969 Aug;15(8):933–940. doi: 10.1139/m69-164. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. High-pressure microbial physiology. Adv Microb Physiol. 1976;14(11):159–241. doi: 10.1016/s0065-2911(08)60228-3. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., ZoBell E. Magnesium and calcium ions enhance barotolerance of Streptococci. Arch Mikrobiol. 1971;79(1):80–92. doi: 10.1007/BF00412043. [DOI] [PubMed] [Google Scholar]
- Pollard E. C., Weller P. K. The effect of hydrostatic pressure on the synthetic processes in bacteria. Bibl Laeger. 1966 Mar 14;112(3):573–580. doi: 10.1016/0926-6585(66)90261-5. [DOI] [PubMed] [Google Scholar]
- Pope D. H., Berger L. R. Inhibition of metabolism by hydrostatic pressure: what limits microbial growth? Arch Mikrobiol. 1973 Nov 19;93(4):367–370. doi: 10.1007/BF00427933. [DOI] [PubMed] [Google Scholar]
- Pope D. H., Smith W. P., Orgrinic M. A., Landau J. V. Protein synthesis at 680 atm: is it related to environmental origin, physiological type, or taxonomic group? Appl Environ Microbiol. 1976 Jun;31(6):1001–1002. doi: 10.1128/aem.31.6.1001-1002.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Goode D., Maugel T. K., Bonar D. B. Pressure-induced depolymerization of spindle microtubules. III. Differential stability in HeLa cells. J Cell Biol. 1976 May;69(2):443–454. doi: 10.1083/jcb.69.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in growing cells of Escherichia coli. Biochem J. 1967 May;103(2):462–466. doi: 10.1042/bj1030462. [DOI] [PMC free article] [PubMed] [Google Scholar]



