Since the publication of Charles Darwin's The Descent of Man and Selection in Relation to Sex in 1871 (1), there has been a vigorous debate about the meaning of sexual dimorphism for a range of physical attributes in numerous animal species, including primates and humans, extinct and extant. Key points of discussion are how to interpret size dimorphism in past humans and human-like ancestors and what inferences can be drawn about the evolution of human mating systems and social organization. In this issue of PNAS, Reno et al. (2) report on their investigation of sexual dimorphism in the three-million-year-old Australopithecus afarensis, an important and well known hominid, ancestral to the genus Homo (3). Insight into dimorphism in this taxon has important implications for social behavior and organization in later and present-day humans.
Body mass dimorphism varies dramatically among primate species, both present and past. For most anthropoids, males are bigger than females (4–8). Humans today display relatively limited sexual dimorphism (≈15%), whereas some of the other hominoids (gorillas and orangutans) are highly dimorphic (>50%) (5, 9). Body mass is easily determined in living species. For past nonhuman primates and human ancestors, mostly represented by fragmentary fossil remains, body mass is far less accessible. Recently, the femur head (the ball of bone at the top of the femur that fits into the hip joint) has been invoked as a source for estimating body mass in early hominids, Homo, and its evolutionary predecessor, Australopithecus (10, 11).
Comparisons of body mass in fossil hominids reveal that general levels of dimorphism have likely remained more or less the same for most of the evolution of Homo, or most of the last two million years to the present (9). In hominids predating Homo, namely the multiple species of Australopithecus, the consensus among paleoanthropologists that has emerged over the last two decades is that pre-Homo species are characterized by high levels of sexual dimorphism (4, 5, 12–15). Close scrutiny of the fossil record, however, suggests that this consensus is built on a data set replete with limitations, especially in regard to reconstructing size dimorphism in Australopithecus.
First, the sample used to estimate dimorphism is very small (fewer than six individuals for A. afarensis). Second, estimates of dimorphism are based on the assumption that sex identification in fragmentary fossil remains used to derive these estimates is accurate. Indeed, the secondary sex characteristics exhibited in the bony pelvis, by far the most reliable of the indicators for humans (16, 17), are largely missing. Thus, investigators are left with size of skeletal elements alone (males have big bones and females have small bones), a poor proxy for pelvic sex identification. Third, accuracy in determination of sexual dimorphism is predicated on correct taxonomic identification. This is especially problematic given that level of sexual dimorphism shows substantial intertaxa variation. Fourth, levels of dimorphism can shift over broad expanses of time (potentially hundreds of thousands of years) or even relatively narrow expanses of time involving hundreds or tens of years (18). Finally, sexual dimorphism levels across broad geographic areas and ecological variation therein may be exaggerated in comparison with contemporary members of a species living in the same place (9).
Reno et al. (2) draw on advances made in statistical modeling to circumvent these limitations of the early hominid fossil record. They apply a new and robust method of simulating dimorphism to an assemblage of A. afarensis representing the remains of individuals who likely died simultaneously in a single catastrophic event some 3.2 million years ago at site A.L. 333, Hadar, Ethiopia. Using the 40% complete skeleton (“Lucy”) from site A.L. 288 as a morphometric template (she has a relatively well preserved femur head and other long bones; Fig. 1), they calculated femoral head diameters from measurements for the postcranial elements from A.L. 333 and other A. afarensis remains. In contrast to the consensus, their analysis revealed only slight to moderate levels of sexual dimorphism, more like Homo and chimpanzees than gorillas.
Fig. 1.
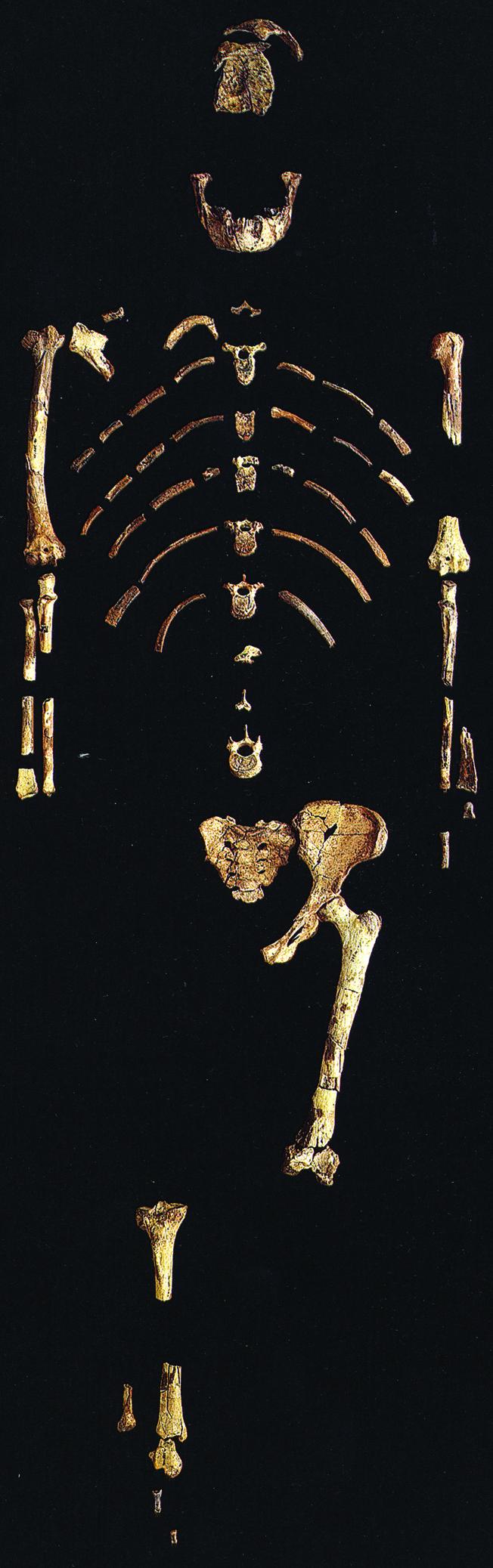
A.L. 288–1 (“Lucy”), the most complete skeleton of Australopithecus afarensis, serves as a morphometric template for determination of sexual dimorphism in other members of the taxon. Original fossil skeleton housed at the National Museum of Ethiopia. Photograph copyright 1985, David L. Brill.
How to interpret this interesting result? By using models derived from study of living nonhuman primates and humans, analysis of sexual dimorphism provides a window onto behavior in earlier hominids and added perspective on the evolution of human social behavior and mating systems. Monomorphic species of living primates (those taxa exhibiting low levels of sexual dimorphism) tend to express minimal male–male competition, whereas dimorphic species tend to express relatively high levels of competition (19–21). Baboon males, for example, are highly intolerant of one another and aggressively compete for access to female mates; simply, success in fights results in greater access to females. For this and other dimorphic primates, sexual selection is only one explanation for high levels of dimorphism, and may not be the best one (22). However, new analyses indicate associations between dimorphism and competition levels (6, 7): where dimorphism is high, male–male competition is commonplace; conversely, where dimorphism is low, competition among males is less frequent.
Although chimpanzee adult males express aggressive behavior toward one another, they tolerate each other, live in multimale kin groups, and are collaborative. Chimpanzee males defend territory and engage in cooperative, coalitionary behavior (23–26).
Perhaps, then, the social organization of A. afarensis might be best characterized as multimale, cooperating (generally noncompeting) kin groups. Based on these new reconstructions of relatively low skeletal dimorphism in A. afarensis, this would seem to be one possible conclusion. However, A. afarensis has lower canine dimorphism than chimpanzees (5–7, 12–14, 27), which suggests a different kind of social organization for these early hominids altogether. The findings of Reno et al. (2) and interpretations based on a range of evidence suggest that A. afarensis had a monogamous and not a polygynous mating system with strong intermale competition as was implied from previous reconstructions of great body size dimorphism. However the data are interpreted, their findings do not contradict what would be expected in a monogamous mating system. Indeed, the relatively low amount of dimorphism is more consistent with pair bonding (and the behaviors associated with it), more so than with the higher levels of dimorphism in single- and multimale extant primate genera (28).
We will never know what the social organization and mating systems were for early hominids; past behaviors do not preserve. However, innovative documentation of morphometric variation in the context of informed study of behavior in living species provides essential perspective on behavior in extinct species. In addition to charting new directions for future analysis, these new findings suggest that earlier behavioral models based on supposedly highly dimorphic pre-Homo taxa are not the most appropriate, and that the earlier consensus about body size dimorphism and its implications requires further discussion. Rather than implying some form of unique behavior based on a combination of low sexual dimorphism in canine size and high sexual dimorphism in body size (5–7, 9), A. afarensis (and other early hominids) may have been more human-like in their basic social behavior. Thus, the roots of human behavior may go deep in time. The article by Reno et al. (2) and the discussion and debate provoked by it will move the field closer to deriving an increasingly informed understanding of sexual dimorphism and social behavior in the remote human past, laying the groundwork for understanding the evolution of human social organization.
Early hominids may have been more human-like in their basic social behavior.
See companion article on page 9404.
References
- 1.Darwin, C. (1871) The Descent of Man and Selection in Relation to Sex (D. Appleton, New York); reprinted (1898) (D. Appleton, New York).
- 2.Reno, P. L., Meindl, R. S., McCollum, M. A. & Lovejoy, C. O. (2003) Proc. Natl. Acad. Sci. USA 100, 9404–9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White, T. D. (2002) in The Primate Fossil Record, ed. Hartwig, W. C. (Cambridge Univ. Press, Cambridge, U.K.), pp. 407–417.
- 4.Frayer, D. W. & Wolpoff, M. H. (1985) Annu. Rev. Anthropol. 14, 429–473. [Google Scholar]
- 5.Plavcan, J. M. (2001) Yearbook Phys. Anthropol. 44, 25–53. [DOI] [PubMed] [Google Scholar]
- 6.Plavcan, J. M. & van Schaik, C. P. (1997) J. Hum. Evol. 32, 345–374. [DOI] [PubMed] [Google Scholar]
- 7.Plavcan, J. M. & van Schaik, C. P. (1997) Am. J. Phys. Anthropol. 103, 37–68. [DOI] [PubMed] [Google Scholar]
- 8.McHenry, H. M. & Coffing, K. (2000) Annu. Rev. Anthropol. 29, 125–146. [Google Scholar]
- 9.Ruff, C. (2002) Annu. Rev. Anthropol. 31, 211–232. [Google Scholar]
- 10.Ruff, C. B. (2000) Am. J. Phys. Anthropol. 113, 507–517. [DOI] [PubMed] [Google Scholar]
- 11.Ruff, C. B., Trinkaus, E. & Holliday, T. W. (1997) Nature 387, 173–176. [DOI] [PubMed] [Google Scholar]
- 12.McHenry, H. M. (1992) Am. J. Phys. Anthropol. 87, 407–431. [DOI] [PubMed] [Google Scholar]
- 13.McHenry, H. M. (1994) J. Hum. Evol. 27, 77–87. [Google Scholar]
- 14.McHenry, H. M. (1994) in Power, Sex, and Tradition: The Archeology of Human Ancestry, ed. Shennan, S. & Steele, J. (Routledge, London), pp. 91–109.
- 15.Richmond, B. G. & Jungers, W. L. (1995) J. Hum. Evol. 29, 229–245. [Google Scholar]
- 16.Krogman, W. M. & Iscan, M. Y. (1986) The Human Skeleton in Forensic Medicine (Thomas, Springfield, IL), 2nd Ed.
- 17.Walker, A. & Ruff, C. B. (1993) in The Nariokotome Homo erectus Skeleton, ed. Walker, A. & Leakey, R. (Harvard Univ. Press, Cambridge, MA), pp. 221–233.
- 18.Ruff, C. B. & Larsen, C. S. (2001) in Bioarchaeology of Spanish Florida: The Impact of Colonialism, ed. Larsen, C. S. (Univ. Press of Florida, Gainesville), pp. 113–145.
- 19.Leigh, S. R. (1997) Am. J. Phys. Anthropol. 97, 339–356. [DOI] [PubMed] [Google Scholar]
- 20.Plavcan, J. M. (2000) J. Hum. Evol. 39, 327–344. [DOI] [PubMed] [Google Scholar]
- 21.Clutton-Brock, T. H. (1985) in Size and Scaling in Primate Biology, ed. Jungers, W. L. (Plenum, New York), pp. 51–60.
- 22.Phillips-Conroy, J. E. & Jolly, C. J. (1981) Am. J. Phys. Anthropol. 56, 115–129. [DOI] [PubMed] [Google Scholar]
- 23.Wrangham, R. & Peterson, D. (1996) Demonic Males: Apes and the Origins of Human Violence (Mariner, Boston).
- 24.Wrangham, R. W. (1999) Yearbook Phys. Anthropol. 42, 1–30. [DOI] [PubMed] [Google Scholar]
- 25.Goodall, J. (1986) The Chimpanzees of Gombe: Patterns of Behavior (Harvard Univ. Press, Cambridge, MA).
- 26.Strier, K. B. (2003) Primate Behavioral Ecology (Allyn & Bacon, Boston), 2nd Ed.
- 27.Leutenegger, W. & Shell, B. (1987) J. Hum. Evol. 16, 359–367. [Google Scholar]
- 28.Harvey, P. H. & Harcourt, A. H. (1984) in Sperm Competition and the Evolution of Animal Breeding Systems, ed. Smith, R. L. (Academic, New York), pp. 589–600.


