Abstract
Branched polyethylenimine (PEI) chains with an average molecular mass of 2 kDa (PEI2) have been covalently attached to gold nanoparticles (GNPs), and the potency of the resulting PEI2–GNPs conjugates as vectors for the delivery of plasmid DNA into monkey kidney (COS-7) cells in the presence of serum in vitro has been systematically investigated. The transfection efficiencies vary as a function of the PEI/gold molar ratio in the conjugates, with the best one (PEI2–GNPII) being 12 times more potent than the unmodified polycation. This potency can be further doubled by adding amphiphilic N-dodecyl–PEI2 during complex formation with DNA. The resulting ternary complexes are at least 1 order of magnitude more efficient than the 25-kDa PEI, one of the premier polycationic gene-delivery vectors. Importantly, although unmodified PEI2 transfects just 4% of the cells, PEI2–GNPII transfects 25%, and the PEI2–GNPII/dodecyl–PEI2 ternary complex transfects 50% of the cells. The intracellular trafficking of the DNA complexes of these vectors, monitored by transmission electron microscopy, has detected the complexes in the nucleus <1 h after transfection.
Gene therapy holds great promise for treating diseases ranging from inherited disorders to acquired conditions and cancer (1–4). The most common vectors (carriers) in current clinical trials are recombinant viruses, wherein the gene of interest is covalently inserted into the viral genome. Unfortunately, such severe side reactions encountered in patients as immune response and insertional mutagenesis leading to death, carcinogenesis, or germ-cell-line alterations pose serious concerns about the clinical application of viral vectors (5–7) and have prompted development of nonviral delivery systems (8–10). Unlike viral vectors, nonviral ones rely on the formation of noncovalent assemblies between DNA (a polyanion) and cationic polymers or liposomes. Vectors based on cationic polymers (polycations) are particularly attractive because of their synthetic maneuverability, allowing incorporation of multiple functional elements within the same molecule without compromising DNA-binding ability (11–13). And yet, available polycations are handicapped by their low potencies (14, 15), prompting the search for new ones with better transfection efficiencies (16–20). It is likely that major improvements may be brought about by elucidating the mechanism of gene delivery by those vectors already relatively competent, followed by their targeted chemical derivatization to overcome extra- and/or intracellular barriers to nonviral gene delivery (10). For these reasons, polyethylenimine (PEI), a synthetic “proton-sponge” polycation (21–24) introduced for transfection a few years ago, is an ideal candidate for such a quest.
The transfection efficiency and toxicity of PEI correlate strongly with its molecular mass. For example, PEI2 is some 2 orders of magnitude less efficient than its 25-kDa counterpart (PEI25) at the same concentration, presumably because of its inability to condense DNA effectively (25). We previously showed that N-alkylating PEI2 with hydrophobic substituents such as dodecyl significantly enhances transfection efficiency, and that N-dodecyl–PEI2 was five times more potent than even PEI25 (24). In the present study we found that PEI2 conjugated to gold nanoparticles (GNPs) transfects COS-7 cells six times more efficiently than PEI25; moreover, the PEI2–GNP conjugates and the N-dodecyl–PEI2 act synergistically, yielding the 11-fold net enhancement in efficiency over PEI25. Importantly, as much as 50% of the cells could be transfected by using the ternary complex, whereas the unmodified PEI2 and PEI25 transfected merely 4% and 8%, respectively.
Experimental Procedures
Materials. The 2- and 25-kDa PEIs, HAuCl4 trihydrate, NaBH4, 4,4′-dithiodibutyric acid, 2-mercaptoethanol, dicyclohexylcarbodiimide, and N-hydroxysuccinimide were purchased from Aldrich. All solvents used, also from Aldrich, were of the highest purity available. Spectra/Por CE dialysis tubing with a molecular mass cutoff of 500 Da was from Spectrum Laboratories (Houston), and cellulose dialysis tubing with a molecular mass cutoff of 12 kDa was from Sigma. Elemental analyses were performed by M-H-W Laboratories (Phoenix). Transmission electron microscopy (TEM) experiments were performed by using a JEOL 1200EX-80kV microscope.
Synthesis of PEI2–GNP Conjugates (Fig. 1). To synthesize PEI2–GNP conjugates, PEI2 was first reacted with the bis-succinimide ester of 4,4′-dithiodibutyric acid. The resulting conjugates were treated with 2-mercaptoethanol to reduce the intra- and inter-molecular disulfides and purified by dialysis (500-Da cutoff). The resulting thiol-modified PEI2 and HAuCl4 were mixed at three different ratios (3:1, 1:3, and 1:10), reduced with 50-mol equivalents (with respect to HAuCl4) of NaBH4, and purified by dialysis (12-kDa cutoff) to obtain the PEI2–GNPs.
Fig. 1.
Schematic representation of the synthetic route toward the conjugates of 2-kDa PEI and GNPs. Step i: conversion of 4,4′-dithiodibutyric acid to its bis-succinimide ester. HOSu, N-hydroxysuccinimide; DCC, dicyclohexylcarbodiimide; DMF, dimethylformamide. Step ii: reduction of the disulfides with 2-mercaptoethanol. Step iii: formation of PEI2–GNP conjugates by the reduction of hydrogen tetrachloroaurate in the presence of the thiol-modified PEI2. The last two reagents were mixed at three different molar ratios, 1:3, 3:1, and 10:1, to obtain the conjugates with different Au/PEI2 ratios (designated PEI2–GNPI, PEI2–GNPII, and PEI2–GNPIII, respectively). See Experimental Procedures for details.
Bis-(N-hydroxysuccinimido)-4,4′-dithiodibutyrate was synthesized according to a modified literature procedure (26): 5.96 g (25 mmol) of 4,4′-dithiodibutyric acid and 7.48 g (65 mmol) of hydroxysuccinimide were dissolved in 25 ml of dimethylformamide at room temperature (used elsewhere henceforth unless stated otherwise), and 11.35 g (55 mmol) of dicyclohexylcarbodiimide dissolved in 20 ml of dimethylformamide was added with stirring. When dicyclohexylurea started precipitating from the reaction mixture, the latter was cooled by using an ice bath, stirred for 12 h, and diluted with ethyl acetate, and the precipitated dicyclohexylurea was filtered off. The ethyl-acetate layer was washed with brine and dried over anhydrous Na2SO4. The solid obtained after evaporation of ethyl acetate was dissolved in methylene chloride and filtered to remove residual dicyclohexylurea. The filtrate was washed three times with saturated potassium carbonate followed by brine and dried over Na2SO4. Evaporation of the solvent furnished the product as a solid (85% yield), and its structure was confirmed by NMR and mass spectral analysis.
Five grams of PEI2 (2.5 mmol) was dissolved in 40 ml of methylene chloride/tetrahydrofuran (5:3) followed by a dropwise addition of 1.0 g of bis-(N-hydroxysuccinimido)-4,4′-dithiodibutyrate (2.31 mmol) dissolved in 15 ml of tetrahydrofuran with stirring over 3 min, followed by a 15-h stirring and evaporation of the solvent to obtain a gel-like, water-insoluble material. The latter was suspended in 50 ml of water, treated with 2.24 ml of 2-mercaptoethanol, and stirred for 2 h. The solution thus obtained was filtered by using a sintered funnel, and the filtrate was dialyzed (500-Da cutoff) extensively against deionized water.
PEI2–GNP conjugates prepared at an initial Au/PEI molar ratio of 1:3 (PEI2–GNPI). Twenty-five milliliters of an aqueous solution of 1.05 g (0.48 mmol) of the thiol-modified PEI2 was mixed with 62.5 mg (0.16 mmol) of HAuCl4·3H2O and stirred for 10 min; then 0.3 g (7.93 mmol) of NaBH4 dissolved in 4 ml of water was added dropwise over ≈40 sec (vigorous reaction), and the mixture was stirred for 24 h. The conjugate thus obtained was dialyzed (12-kDa cutoff) extensively against water deionized with the Milli-Q system (used elsewhere henceforth unless specified otherwise), and the dialyzate was diluted to 100 ml to obtain a stock solution. A measured portion of the latter was lyophilized, and its elemental analysis yielded the gold content of 19.4% (Au/PEI molar ratio = 3.0). The total amount of PEI present in the stock solution was calculated from the weight of the solid obtained after lyophilization and the elemental analysis data.
PEI2–GNP conjugates prepared at an initial Au/PEI molar ratio of 3:1 (PEI2–GNPII). Twenty-five milliliters of an aqueous solution of 1.05 g (0.48 mmol) of the thiol-modified PEI2 in water was mixed with 562 mg (1.43 mmol) of HAuCl4·3H2O and stirred for 10 min; then 2.7 g (71.4 mmol) of NaBH4 dissolved in 36 ml of water was added dropwise over ≈90 sec (vigorous reaction), and the mixture was stirred for 24 h. The conjugate thus obtained was dialyzed extensively against water (12-kDa cutoff), and the dialyzate was diluted to 200 ml to obtain a stock solution. A measured portion of the latter was lyophilized, and its elemental analysis yielded the gold content of 57.8% (Au/PEI molar ratio = 15). The total amount of PEI present in the stock solution was calculated as in the case of PEI2–GNPI.
PEI2–GNP conjugates prepared at an initial Au/PEI molar ratio of 10:1 (PEI2–GNPIII). Twenty-five milliliters of an aqueous solution of 0.53 g (0.24 mmol) of the thiol-modified PEI2 in water was mixed with 938 mg (2.38 mmol) of HAuCl4·3H2O and stirred for 2 min; then 4.5 g (119.0 mmol) of NaBH4 dissolved in 36 ml of water was added dropwise over 90 sec (vigorous reaction), and the mixture was stirred for 24 h. The resulting mixture that, unlike in the previous two instances, also contained water-insoluble nanoparticles was filtered by using a 0.2 μM pore polyether sulfone membrane (Nalge filter unit), and the filtrate was dialyzed. The conjugates thus obtained were dialyzed extensively against water (12-kDa cutoff), and the dialyzate was diluted to 200 ml to obtain a stock solution. A measured amount of the latter was lyophilized, and its elemental analysis yielded the gold content of 80.0% (Au/PEI molar ratio = 44.6). The total amount of PEI present in the stock solution was calculated as described above.
Plasmid. gWiz Beta-Gal (8,278 bp) encoding the β-galactosidase (β-gal) gene was purchased from Aldevron (Fargo, ND). This plasmid contains the β-gal gene under the control of a modified promoter from the cytomegalovirus immediate early gene. This ready-to-use reporter plasmid was obtained as a 1.0 mg/ml stock solution in aqueous Tris·HCl/EDTA buffer.
Cell Culture and Transfection. COS-7 cells (simian virus 40-transformed kidney cells of an African green monkey) were cultured in DMEM-containing Gln supplemented with 10% heat-inactivated FBS (both from GIBCO) and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin from Sigma). Cells were grown at 37°C in humidified air containing 5% CO2 and passaged every 3–4 days. The cells (3 × 105 per well) were plated on Costar six-well tissue-culture clusters 24 h before transfection. Immediately before the initiation of transfection experiments, the medium was removed from each well, and the cells were washed once with DMEM without serum and antibiotics and treated with the polyplexes as described below.
In each well, 2.5 μg of the plasmid DNA (gWiz Beta-Gal) was used. Polyplexes were formed in phosphate-buffered saline containing glucose unless otherwise specified. The quantities of plasmid and PEIs given below correspond to experiments done in triplicate.
Stock solutions of the PEI2–GNPs were stored at 4°C, brought to room temperature, and diluted with water before complex formation with DNA. Stock solutions of PEI2, PEI25, and N-dodecyl–PEI2 were prepared at 100 mM concentration (with respect to PEI nitrogens) in water and diluted appropriately with water. The term “polycation” refers to both PEIs and PEI2–GNPs. N/P ratios refer to the ratio of PEI nitrogen to DNA phosphate.
The polyplexes were prepared by the addition, with stirring, of appropriate amounts of the polycation in 300 μl of water to 7.5 μg of plasmid DNA in 300 μl of 20 mM aqueous PBS containing 10% glucose. The resulting solutions were incubated at 37°C for 25 min and diluted to 7.5 ml with DMEM with 10% FBS. To each well, 2.5 ml of this transfection medium was added, followed by incubation at 37°C in a humidified-air (5% CO2) atmosphere for 6 h. The transfection medium then was removed, and the cells were incubated further under the same conditions in a complete medium (3 ml per well) for 42 h. Thereafter, the medium was removed from each well, the cells were washed twice with Dulbecco's PBS without CaCl2 and MgCl2 (Sigma) and lysed with 560 μl of Reporter lysis buffer (Promega) following manufacturer protocol, and the lysates were assayed for β-gal activity spectrophotometrically by monitoring the absorbance of o-nitrophenolate at 420 nm (27). The results were expressed as relative β-gal activity per mg of protein. Total protein was estimated from the bicinchoninic acid (Sigma) assay (28).
5-Bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) Staining of Transfected Cells. After 48 h of transfection performed as described above, the medium was removed, and the cells were fixed with 0.05% glutaraldehyde (Sigma) in 2 ml of aqueous PBS for 5 min. The buffer containing the fixative was removed, and the cells were washed three times with 2 ml of aqueous PBS, with a 5-min incubation during the second washing. Then, 2 ml of a 1 mg/ml X-Gal (Sigma) solution containing 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 1 mM MgCl2 in PBS was added to the cells and incubated at 37°C/5% CO2 for 24 h (29). The X-Gal solution was removed, and the cells were washed with 2 ml of aqueous PBS, incubated with 2 ml of the fresh buffer, and examined under a microscope.
TEM. Carbon-coated Cu grids (300-mesh) were used in all experiments. For TEM involving dodecyl–PEI2, 10 μl of the stock solution in water was kept on a grid for 2 min, excess solution was blotted off by using filter paper, and the grids were stained with 5 μl of 1% aqueous uranyl acetate for 30 sec. Excess staining solution was blotted off by using filter paper, and the grids were air-dried. In the case of PEI2–GNP conjugates, 10 μl of the stock solution in water was kept on a grid for 2 min, excess solution was blotted off by using filter paper, and the grids were air-dried. PEI2–GNPII/plasmid DNA complexes for TEM were prepared in Hepes-buffered saline (HBS) buffer (molecular biology grade, Fluka). Complexes were prepared by adding 100 μl of the PEI2–GNPII solution in water to 100 μl of 2× HBS buffer containing 2.5 μg of plasmid DNA, followed by incubation at 37°C for 25 min. Ten microliters of this solution in water was kept on a grid for 2 min, excess solution was blotted off by using filter paper, and the grids were air-dried.
To monitor the intracellular trafficking of the complexes, COS-7 cells were transfected as described above. Forty minutes after the transfection, the medium was removed, and the cells were washed twice with HBS, incubated with 750 μl of 0.1 M Sorensen's phosphate buffer, scraped gently, and transferred to Eppendorf tubes. This cell suspension was mixed with an equal volume of 5% glutaraldehyde in the same buffer, fixed for 30 min, and pelleted. The cell pellets were washed with HBS and water, postfixed with 1% aqueous OsO4 (Fluka) for 1 h, pelleted, and washed again twice with HBS. The cells then were dehydrated through ethanol series (70% for 15 min, 90% for 15 min, and 100% for 15 min twice) and embedded in Epon/Araldite resin (polymerization at 65°C for 15 h). Thin sections (90-nm) containing the cells were placed on the grids and stained for 1 min each with 4% uranyl acetate (1:1, acetone/water) and 0.2% Raynolds lead citrate (water), air-dried, and examined under the transmission electron microscope (30).
Cytotoxicity Measurements. Cell culture and transfection were performed under the same conditions as outlined above. Control cells were treated with 2.5 ml per well of DMEM containing 200 μl of phosphate-buffered saline containing glucose, whereas other cells were treated with either the plasmid or the polyplexes. Cytotoxicities were evaluated by measuring the metabolic activity of the cells 48 h posttransfection by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (31) as described (24). The results are expressed as percentages relative to control cells (mean ± SD, n = 3).
Results and Discussion
Although a leading class of synthetic gene-delivery vehicles, polycations suffer from low efficiencies compared with those of recombinant viral vectors (14, 15). Improvements may be achieved by chemically modifying a given polycation to enhance one or more steps involved in the polyplex-mediated gene delivery, additionally generating an insightful structure–efficiency relationship along the way (10). We selected PEIs for such an investigation because of their superior performance compared with other nonviral vectors, low cost, and amenability to diverse and selective chemical modifications. The transfection efficiency and toxicity of PEI strongly correlate with its molecular weight. For example, PEI2 is some 2 orders of magnitude less efficient, as well as noncytotoxic, compared with PEI25 (at N/P = 10, where the latter is most efficient and moderately cytotoxic). Previously we had shown that attaching hydrophobic substituents, e.g., dodecyl groups, to PEI2 enhances its transfection efficiency >100-fold (24). Our subsequent TEM examination revealed that dodecyl–PEI2 self assembles in water. Coupled with the efficient transfection of dodecyl–PEI2 at relatively low N/P ratios (20 for maximum efficiency), this finding indicates that the DNA-binding entities are the agglomerated dodecyl–PEI2s rather than the individual molecules. In other words, self-assembly mediated by the hydrophobic interactions between the dodecyl substituents leads to a greater effective molecular weight and thus more efficient DNA condensation. Additionally, the hydrophobic substituents would also benefit the interaction of the polyplexes with plasma membrane and with endosomal-lysosomal membrane components, thus influencing cellular uptake and endosomal escape. All these factors could synergistically contribute to the observed enhancement in efficiency.
Prompted by the aforementioned observations, we set out to explore alternative means of generating novel efficient PEI2-based gene-delivery vectors. In the present work we report the effect of covalent attachment of PEI2 to GNPs on the transfection efficiency of the polycation in the presence of serum in vitro in COS-7 cells. The underlying premise of this study is that conjugating PEI2 to GNPs would increase its effective molecular weight, consequently enhance DNA binding and condensation, and therefore improve transfection. GNPs seemed appealing because of their biocompatibility, optical properties, high electron density, and ease of conjugation to biomolecules. In fact, for these reasons GNPs are actively investigated for various bioanalytical and biomedical applications (32–38); for example, due to the high electron density of gold, the cellular trafficking of GNP bioconjugates is readily monitored by TEM (37, 38).
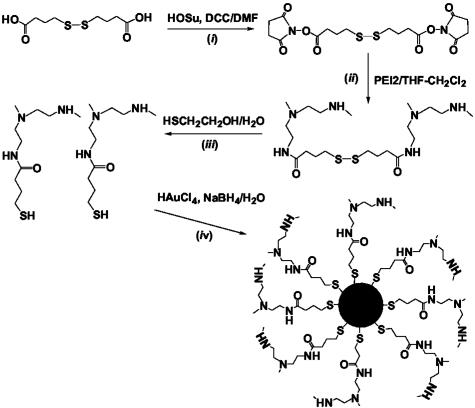
PEI2–GNP conjugates were synthesized by reducing hydrogen tetrachloroaurate with sodium borohydride (39–42) in the presence of the thiol-modified PEI2 (Fig. 1). To identify the optimal conjugates in terms of transfection efficiency, they were prepared at three different PEI2/Au molar ratios. The resultant ratios in the purified conjugates were proportional to the initial ones (see Experimental Procedures), as observed with other ligands (41, 42). All the conjugates exhibited the plasmon band of GNPs with λmax ≈ 500 nm (Fig. 2A). The sizes of the gold core deduced from TEM were 4.1 ± 0.9 nm for PEI2–GNPI, 2.3 ± 0.9 nm for PEI2–GNPII, and 3.7 ± 1.5 nm for PEI2–GNPIII. An electron micrograph of PEI2–GNPII and that of its complex with the plasmid DNA are presented in Fig. 2 B and C, respectively.
Fig. 2.
Absorption spectra of the PEI2–GNP conjugates (A) and electron micrographs of PEI2–GNPII conjugates (B) and the polyplex of PEI2–GNPII with the plasmid DNA (gWiz Beta-Gal) formed at N/P = 150 (C). See Experimental Procedures for details.
Initial transfection experiments were performed with all three PEI2–GNPs. Polyplexes with N/P ratios ranging from 2.5 to 210 were included in this initial screening, and the optimal N/P ratio in each case was determined. The transfection efficiencies varied as a function of the PEI/Au molar ratio in the conjugates and declined in the order PEI2–GNPII > PEI2–GNPI > PEI2–GNPIII (measured at the respective optimal N/P ratios of 150, 90, and 60). Specifically, PEI2–GNPI and PEI2–GNPIII were some 12 and 155 times less efficient, respectively, than PEI–GNPII. Polyplexes of PEI2–GNPI formed aggregates visible to the naked eye, a likely cause of the reduced transfection efficiency. Polyplexes of PEI2–GNPII and PEI2–GNPIII exhibited no apparent signs of aggregation. Because PEI2–GNPII exhibited the highest efficiency among the three conjugates, it was selected for all subsequent experiments.
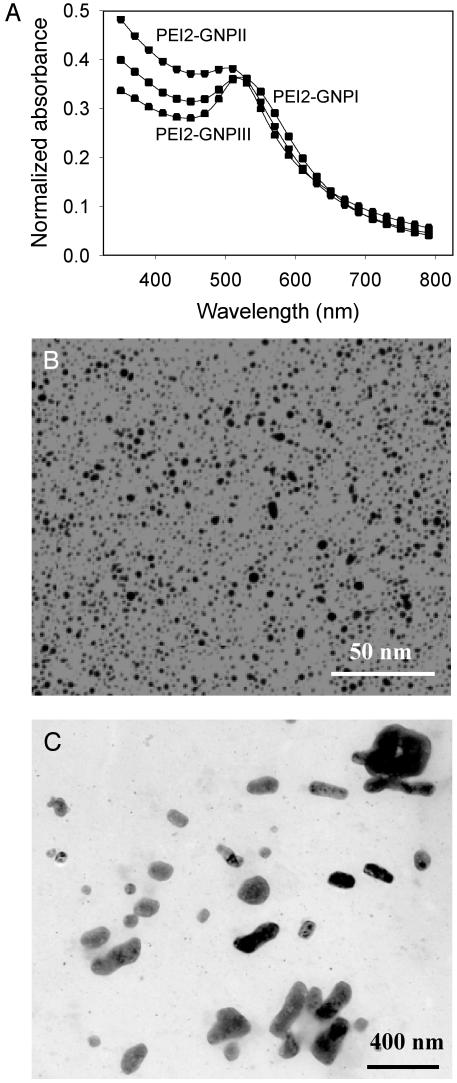
The potency of PEI2–GNPII was compared systematically with those of the parent PEI2 as well as PEI25. At the N/P ratios of 90, 120, and 150 (Fig. 3A, bars a–c), the conjugates (Fig. 3A, bars d–f) exhibited, respectively, 15, 14, and 12 times higher transfection efficiencies than PEI2. Moreover, at an N/P of 150 (Fig. 3B, bar d), PEI2–GNPII was six times more efficient than PEI25 at N/P = 10 (Fig. 3B, bar a). (It is worth mentioning that at N/P = 150, the efficiency of the unmodified PEI2 was approximately half that of PEI25 at N/P = 10.)
Fig. 3.
(A) Extents of expression in COS-7 cell cultures of the β-gal gene mediated in the presence of serum by PEI2 (bars a–c) and PEI2–GNPII (bars d–f) at different N/P ratios. Bars a and d, N/P = 90; bars b and e, N/P = 120; bars c and f, N/P = 150. (B) Extents of expression in COS-7 cell cultures of the β-gal gene mediated in the presence of serum by PEI25 (bar a), dodecyl–PEI2 (bars b and c), PEI2–GNPII (bar d), and PEI2–GNPII + dodecyl–PEI2 (bars e and f). Bar a, N/P = 10; bar b, N/P = 20; bar c, N/P = 40; bar d, N/P = 150; bar e, N/P = 150 + 20; bar f, N/P = 150 + 40. Cells transfected with the plasmid in the absence of polycations showed no appreciable β-gal activity.
Next, we examined whether the efficiency of PEI2–GNPII could be enhanced further by complexation with dodecyl–PEI2 (it was reasoned that an enhancement in the hydrophobicity of the complex could have a beneficial effect on the cellular uptake of the complexes). To this end, dodecyl–PEI2 was coincubated with PEI2–GNPII (N/P = 150) at two N/P ratios of the former, 20 and 40. Interestingly, the efficiency of the ternary complexes (Fig. 3B, bars e and f) was found to far exceed those of both PEI2–GNPII alone (Fig. 3B, bar d) and dodecyl–PEI2 alone (Fig. 3B, bars b and c). Similar enhancements were observed irrespective of the addition order: the polycations could be either added first to DNA or premixed and then added to DNA. The efficiency of the ternary complex formed by PEI2–GNPII (N/P = 150) with dodecyl–PEI2 (N/P = 40) was also 11-fold higher than that of PEI25 (Fig. 3B, bar a).
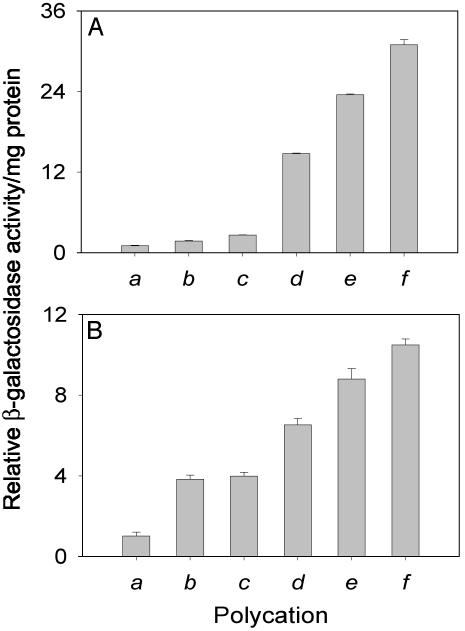
The fractions of the cells transfected by the unmodified PEIs, PEI2–GNPII, and the ternary complex PEI2–GNPII + dodecyl–PEI2 were independently assessed by histochemical staining by using X-Gal. Although PEI2 (N/P = 150) and PEI25 (N/P = 10) transfected merely 4% and 8% of the cells, respectively, PEI2–GNPII (N/P = 150) transfected some 25% and PEI2–GNPII + dodecyl–PEI2 (N/P = 150 + 40) as much as 50% of the cells (Fig. 4). That the enhanced global transfection efficiency afforded by PEI2–GNPII and PEI2–GNPII/dodecyl–PEI2 also resulted in a concomitant increase in the number of transfected cells is especially significant for such applications as gene delivery to cancer cells, where a higher percentage of transfected cells is crucial.
Fig. 4.
Histochemical staining with X-Gal of COS-7 cell cultures transfected in the presence of 10% serum with the β-gal gene mediated by PEI2 and its derivatives: PEI2 (N/P = 150) (A), PEI2–GNPII (N/P = 150) (B), and PEI2–GNPII + dodecyl–PEI2 (N/P = 150 + 40) (C).
The cytotoxicities of the complexes of the various PEI-based polycations are presented in Table 1. It is seen that ≈80% of the cells were still metabolically active in the case of PEI2–GNPII complexes, whereas in the case of the ternary complex of PEI2–GNPII with dodecyl–PEI2, the percentage of metabolically active cells decreased to ≈70%. The reasons for the moderate cytotoxicity induced by polyplexes are not well understood. In the case of PEI800, micrometer-size complexes were detected on the surface of the plasma membrane (43, 44). It was suggested that the deposition of such clusters might impair the plasma-membrane functions and lead to cell death. This explanation is unlikely to hold in the present case, because the complexes found to be associated with the plasma membrane and those inside the cells were far smaller (100–300 nm). An additional source of induction of cellular dysfunction and cytotoxicity may arise from the ability of PEI to enter the cell nucleus (see below).
Table 1. Cytotoxicity to COS-7 cells of polyplexes formed by PEI2 and its GNP conjugates with plasmid DNA.
| Polycation | Cell viability, % of control |
|---|---|
| None | 101 ± 3 |
| PEI2* | 93 ± 5 |
| PEI2† | 92 ± 2 |
| PEI2‡ | 93 ± 2 |
| PEI2-GNPII* | 78 ± 2 |
| PEI2-GNPII† | 78 ± 2 |
| PEI2-GNPII‡ | 76 ± 2 |
| PEI2-GNPII‡§ | 69 ± 1 |
| PEI2-GNPII‡¶ | 67 ± 2 |
Cytotoxicity was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The viability of the nontransfected cells in the absence of PEI is taken as 100%. See Experimental Procedures for additional details.
N/P = 90.
N/P = 120.
N/P = 150.
Ternary complex of PEI2-GNPII with dodecyl-PEI2 (N/P = 20).
Ternary complex of PEI2-GNPII with dodecyl-PEI2 (N/P = 40).
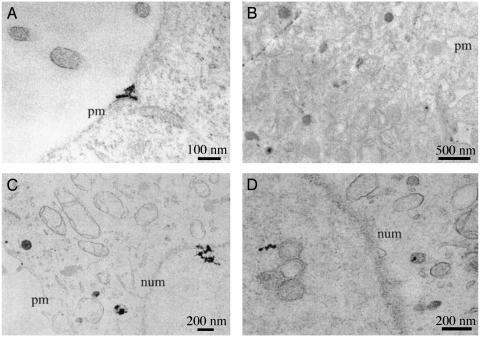
The intracellular trafficking of PEI2–GNPII/DNA and PEI2–GNPII/dodecyl–PEI2/DNA polyplexes could be readily monitored by TEM. Cells were fixed 40 min after the initiation of transfection, and representative electron micrographs taken are presented in Fig. 5. Polyplexes are seen outside the cells, associated with the plasma membrane (Fig. 5A), inside the cytoplasm (Fig. 5B), entering the nucleus (Fig. 5C), and also inside the nucleus (Fig. 5D). Nuclear entry of polyplexes as early as 40 min after transfection signifies that active cell division is not important for this process. Nuclear entry of polycations, on the other hand, would have implications for the toxicity induced by the polyplexes. For instance, PEI may interact with the nuclear DNA and alter the transcriptional process. Although polyplexes were observed for both PEI2–GNPII and PEI2–GNPII/dodecyl–PEI2, a larger percentage of the cells had them in their nuclei in the latter case, wherein higher transfection efficiency and slightly higher toxicity were also observed.
Fig. 5.
Intracellular trafficking in COS-7 cell cultures of the polyplexes of PEI2–GNPII (A and B) and PEI2–GNPII + dodecyl–PEI2 (C and D) with plasmid DNA monitored by TEM 40 min after transfection. (A) Polyplexes are outside the cell and also being endocytosed. (B) Polyplexes have already been endocytosed. (C) Polyplexes entering the nucleus. (D) Polyplexes have entered the nucleus. Plasma membrane (pm) and nuclear membrane (num) are marked.
In closing, several salient features pertinent to polycation-based gene delivery were ascertained herein by using a low-molecular-weight PEI conjugated to GNPs. As a result, a PEI2–GNP conjugate that exhibits six times higher transfection efficiency compared with even the “gold-standard” PEI25 has been prepared. The efficiency of this conjugate could be augmented further by complexation with hydrophobic dodecyl–PEI2. Importantly, with such ternary complexes as much as half of all the cells present could be transfected, whereas the parent PEI2 transfected only 4%. In addition to resulting in a polycationic system for more efficient transfection in vitro, this study also reveals the synergetic action of two modified low-molecular-weight PEIs. This finding implies that this polycation may be also modified independently to incorporate such functions as specific cell-type or nuclear targeting and then complexed with DNA to obtain “multicomponent polyplexes” with superior performance.
Acknowledgments
We are grateful to Professor Ram Sasisekharan for allowing us to use his tissue-culture facility. This work was financially supported by the Biotechnology Process Engineering Center at the Massachusetts Institute of Technology and National Institutes of Health Grant GM26698.
Abbreviations: PEI, polyethylenimine; GNP, gold nanoparticle; TEM, transmission electron microscopy; β-gal, β-galactosidase; N/P, ratio of PEI nitrogen to DNA phosphate; X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactoside.
References
- 1.Wang, L., Takabe, K., Bidlingmaier, S. M., Ill, C. R. & Verma, I. M. (1999) Proc. Natl. Acad. Sci. USA 96, 3906–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frater, A. J., Fidler, S. J. & McClure, M. O. (2002) Gene Ther. 9, 189–213. [Google Scholar]
- 3.McNeish, I. & Seckl, M. J. (2002) Gene Ther. 9, 87–134. [Google Scholar]
- 4.McCormick, F. (2001) Nat. Rev. Cancer 1, 130–140. [DOI] [PubMed] [Google Scholar]
- 5.Marshall, E. (2000) Science 286, 2244–2245. [DOI] [PubMed] [Google Scholar]
- 6.Check, E. (2002) Nature 420, 116–118. [DOI] [PubMed] [Google Scholar]
- 7.Boyce, N. (2001) Nature 414, 677–678. [DOI] [PubMed] [Google Scholar]
- 8.Miller, A. D. (1998) Angew. Chem. Int. Ed. Engl. 37, 1768–1785. [Google Scholar]
- 9.Davis, M. E. (2002) Curr. Opin. Biotechnol. 13, 128–131. [DOI] [PubMed] [Google Scholar]
- 10.Thomas, M. & Klibanov, A. M. (2003) Appl. Microbiol. Biotechnol. 62, 27–34. [DOI] [PubMed] [Google Scholar]
- 11.Merlin, J.-L., N′Doye, A., Bouriez, T. & Dolivet, G. (2002) Drug News Perspect. 15, 445–451. [DOI] [PubMed] [Google Scholar]
- 12.Liu, W. G., Kang, D. Y. & Liu, Q. G. (2001) J. Appl. Polym. Sci. 82, 3391–3395. [Google Scholar]
- 13.Varga, C. M., Wickham, T. J. & Lauffenburger, D. A. (2000) Biotechnol. Bioeng. 70, 593–605. [DOI] [PubMed] [Google Scholar]
- 14.Diebold, S. S., Lehrmann, H., Kursa, M., Wagner, E., Cotton, M. & Zenke, M. (1999) Hum. Gene Ther. 10, 775–786. [DOI] [PubMed] [Google Scholar]
- 15.Boussif, O., Zanta, M. A. & Behr, J.-P. (1996) Gene Ther. 3, 1074–1080. [PubMed] [Google Scholar]
- 16.Read, M. L., Bremner, K. H., Oupicky, D., Green, N. K., Searle, P. F. & Seymour, L. W. (2003) J. Gene Med. 5, 232–245. [DOI] [PubMed] [Google Scholar]
- 17.Akinc, A., Lynn, D. M., Anderson, D. G. & Langer, R. (2003) J. Am. Chem. Soc. 125, 5316–5323. [DOI] [PubMed] [Google Scholar]
- 18.Oupicky, D., Parker, A. L. & Seymour, L. W. (2002) J. Am. Chem. Soc. 124, 8–9. [DOI] [PubMed] [Google Scholar]
- 19.Balicki, D., Putnam, C. D., Scaria, P. V. & Beutler, V. (2002) Proc. Natl. Acad. Sci. USA 99, 7467–7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joester, D., Losson, M., Pugin, R., Heinzelmann, H., Walter, E., Merkle, H. P. & Diederich, F. (2003) Angew. Chem. Int. Ed. Engl. 42, 1486–1490. [DOI] [PubMed] [Google Scholar]
- 21.Boussif, O., Lezoualc'h, F., Zanta, A., Mergny, M. D., Scherman, D., Demeneix, B. & Behr, J.-P. (1995) Proc. Natl. Acad. Sci. USA 92, 7297–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pichon, C., Concalves, C. & Midoux, P. (2001) Adv. Drug Delivery Rev. 53, 75–94. [DOI] [PubMed] [Google Scholar]
- 23.Kichler, A., Leborgne, C., März, J., Danos, O. & Burkhard, B. (2003) Proc. Natl. Acad. Sci. USA 100, 1564–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas, M. & Klibanov, A. M. (2002) Proc. Natl. Acad. Sci. USA 99, 14640–14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen, H., Kunath, K., Martin, A. L., Stolnik, S., Roberts, C. J., Davies, M. C. & Kissel, T. (2002) Biomacromolecules 3, 926–936. [DOI] [PubMed] [Google Scholar]
- 26.Smith, P. K. (1976) U.S. Patent 3,940,420.
- 27.Alam, J. & Cook, J. L. (1990) Anal. Biochem. 188, 245–254. [DOI] [PubMed] [Google Scholar]
- 28.Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J. & Klenk, D. C. (1985) Anal. Biochem. 150, 76–85. [DOI] [PubMed] [Google Scholar]
- 29.Cho, C.-W., Cho, Y.-S., Lee, H.-K., Yeom, Y.-I., Park, S.-N. & Yoon, D.-Y. (2000) Biotechnol. Appl. Biochem. 32, 21–26. [DOI] [PubMed] [Google Scholar]
- 30.Joshee, N., Bastola, D. R. & Cheng, P.-W. (2002) Hum. Gene Ther. 13, 1991–2004. [DOI] [PubMed] [Google Scholar]
- 31.Hansen, M. B., Nielsen, S. E. & Berg, K. (1989) J. Immunol. Methods 119, 203–210. [DOI] [PubMed] [Google Scholar]
- 32.Niemeyer, C. M. & Ceyhan, B. (2001) Angew. Chem. Int. Ed. Engl. 40, 3685–3688. [DOI] [PubMed] [Google Scholar]
- 33.Ma, Z., Liang, R., Jiang, W., Zhou, T., Chen, Z., Duan, M., Tang, J. & Sui, S.-F. (2002) Chem. Lett., 570–571.
- 34.Sandhu, K. K., McIntosh, C. M., Simard, J. M., Smith, S. W. & Rotello, V. M. (2002) Bioconjugate Chem. 13, 3–6. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell, D. J., Taylor, J. R. & Nie, S. (2002) J. Am. Chem. Soc. 124, 9606–9612. [DOI] [PubMed] [Google Scholar]
- 36.Tkachenko, A. G., Xie, H., Coleman, D., Glomm, W., Ryan, J., Anderson, M. F., Franzen, S. & Feldheim, D. L. (2003) J. Am. Chem. Soc. 125, 4700–4701. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, J. M., Takizawa, T. & Vandre, D. D. (2000) J. Microsc. (Oxford) 199, 163–179. [DOI] [PubMed] [Google Scholar]
- 38.Lin, C.-C., Yeh, Y.-C., Yang, C.-Y., Chen, C.-L., Chen, G.-F., Chen, C.-C. & Wu, Y.-C. (2002) J. Am. Chem. Soc. 124, 3508–3509. [DOI] [PubMed] [Google Scholar]
- 39.Chechik, V. & Crooks, R. M. (1999) Langmuir 15, 6364–6369. [Google Scholar]
- 40.Shon, Y.-S., Wuelfing, W. P. & Murray, R. W. (2001) Langmuir 17, 1255–1261. [Google Scholar]
- 41.Hosteler, M. J., Wingate, J. E., Zhong, C.-J., Harris, J. E., Vachet, R. W., Clark, M. R., Londono, J. D., Green, S. J., Stokes, J. J., Wignall, G. D., et al. (1998) Langmuir 14, 17–30. [Google Scholar]
- 42.Yonezawa, T., Yasui, K. & Kimizuka, N. (2001) Langmuir 17, 271–273. [Google Scholar]
- 43.Fischer, D., Bieber, T., Li, Y., Elsasser, H.-P. & Kissel, T. (1999) Pharm. Res. 16, 1273–1279. [DOI] [PubMed] [Google Scholar]
- 44.Bieber, T., Meissner, W., Kostin, S., Niemann, A. & Elsasser, H.-P. (2002) J. Controlled Release 82, 441–454. [DOI] [PubMed] [Google Scholar]