Abstract
The generation of plasmin by plasminogen (Pg) activators (PAs) is a physiologic process in animals that dissolves blood clots and promotes wound healing, blood vessel growth, and the migration of normal and cancerous cells. Pathogenic bacteria have evolved PAs [e.g., streptokinase (SK) and staphylokinase] that exploit the Pg system to infect animals. Animal PAs have a conserved ability to cleave a wide spectrum of animal Pgs, but the ability of bacterial PAs to cleave different animal Pgs is surprisingly restricted. We show that the spectrum of activity of an archetypal bacterial PA (SK) with animal Pgs can be profoundly altered by mutations that affect intermolecular complementarity at sites that participate in complex formation or substrate binding. Comparative sequence analysis of animal plasmins vs. close structural homologues (trypsin and chymotrypsin) that are not molecular targets for invading bacteria indicates that the sites in plasmin that interact with SK are preferentially targeted for mutation. Conversely, intermolecular contact sites in SKs that activate human Pg are more highly conserved than other loci in the molecule or than the same sites in other SKs that activate non-human Pgs. We propose that active modulation of intermolecular complementarity at sites of contact between SK and Pg may represent a competitive evolutionary strategy in a survival battle, whereby animals seek to evade bacterial invasion, and bacteria endeavor to invade their animal hosts.
The generation of plasmin by plasminogen (Pg) activators (PAs) is a physiologic process in animals that dissolves blood clots and promotes wound healing, blood vessel growth, and the migration of normal and cancerous cells (1, 2). Plasmin is generated when PAs cleave the Arg-561-Val bond of the proenzyme Pg (3). Animal PAs (tissue PA and urinary-type PA or urokinase) are serine proteases whose function is tightly regulated by inhibitors, cofactors (fibrin and receptors), and other mechanisms (2). Although the average overall sequence identity among Pgs is 86 ± 7% (to human Pg), mammalian PAs appear to be able to activate a broad spectrum of animal Pgs, which indicates that these protein–protein interactions are conserved. This conservation of functional protein–protein interactions between molecules may be due to a process of mutual coevolution of interaction partners whereby mutations in one molecule of an interacting pair are constrained by the need to maintain an efficient interaction with the partner (4–6).
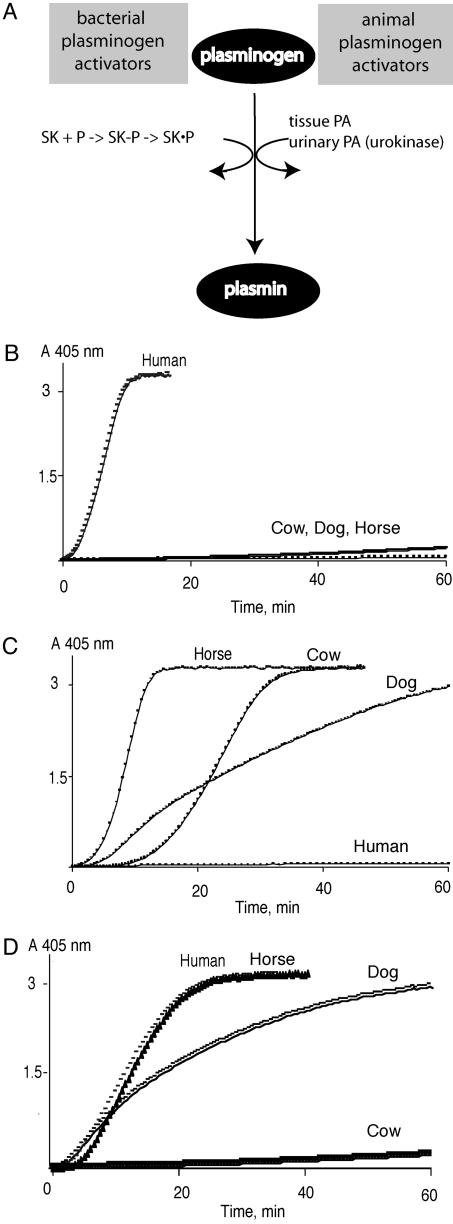
The molecular pathogenesis of infections is complex (reviewed in ref. 7). Proteolysis is one factor that facilitates certain types of infections. The broad proteolytic activity of plasmin and the high concentration of Pg in animals make the Pg system an attractive target for pathogenic bacteria to exploit. The generation of plasmin enhances the virulence of certain Streptococcus sp. and other bacteria in animal models of infection (1, 8–12). Some bacterial species that bind PA produce the potent prokaryotic Pg activators–streptokinase (SK) and staphylokinase. In contrast to animal PAs, bacterial PAs are enzymatically inert but serve as cofactors with plasmin to produce functional “activator complexes.” These bacterial PAs subvert the normal physiologic control mechanisms of the Pg system. For example, SK converts plasmin from an enzyme that cleaves fibrin clots to an enzyme that cleaves Pg (13). In contrast to the fibrin- and cell-targeted action of animal PAs, SK generates plasmin in solution and, makes plasmin in the SK complex resistant to its major inhibitor α2-antiplasmin (13–15). To accomplish these functions, the three domains of SK have extensive intermolecular interactions with PA that include: (i) binding and formation of an SK–plasmin enzyme complex that is kinetically distinguishable from plasmin; and (ii) creation of binding site for Pg substrate to be cleaved by the SK-plasmin complex (Fig. 1A) (16).
Fig. 1.
Pg activation. (A) Schema of Pg activation by bacterial and animal PAs. SK initially binds to plasmin to form a complex (SK-P) with altered enzymatic properties. SK can also bind to Pg and convert it to an enzyme. In the SK–plasmin complex (SK·P), SK binds Pg substrate for Pg activation. Tissue-type PA and urinary-type PA (including urokinase) are serine proteases that directly cleave Pg substrate to form plasmin. (B–D) Activation of cow, human, horse, and dog Pgs (300 nM) by a fixed concentration (20 nM) of SK (B), SUPA (C), or SKβswap (D)at 37°C. Plasmin production was monitored at 405 nm by using a selective substrate (S2251, H-d-valyl-l-leucyl-l-lysine-p-nitroanilide dihydrochloride, Chromogenix, Molndal, Sweden) (0.5 mM) in 50 mM Tris·HCl (pH 7.4)/100 mM NaCl.
Surprisingly, although the ability of their animal PAs to cleave a wide spectrum of animal Pgs (17) has been conserved, the restricted spectrum of function of bacterial PAs with animal Pgs has been a mystery since its initial description 80 years ago (8, 18–23). Indeed, the restricted activity of SK with different animal Pgs may explain why streptococcal isolates cause disease only in a limited number of mammalian hosts. Although the basic principles of Pg activation by SK are now known, and the SK-microplasmin complex structure has been solved (16), the nature and mechanism of species-restricted action of SK are poorly understood. Molecular insights into the mechanism of this species-restricted Pg activation may help elucidate the basis for species-restricted infection and facilitate the design of new blood clot-dissolving drugs.
Materials and Methods
Cloning, Expression, Purification, and Titration of Recombinant Proteins. Recombinant Streptococcus uberis PA (SUPA), SK, SK α-SUPA β-SK γ chimera (SKβswap), and micro-Pg were cloned, expressed in bacteria, purified, and characterized as described (13, 15, 21–23). Human micro-Pg was chimerized at loops 5, 7, 9, and 11 with the corresponding loop sequences of bovine micro-Pg by the PCR with overlap extension by using the following sense and antisense primers, respectively: loop 5-gacaacattttagcgctgtcattctacaaggtcatcctgggtgc and gaatgacagcgctaaaatgttgtccaagcagtgggcagcagtc; loop 7-ccctcacaggcagatattgccttgc and gcaaggcaatatctgcctgtgaggg; loop 9-ggtacttttggagagggccttctcaagg and ccttgagaaggccctctccaaaagtacc; and loop 11-ctggacggaagagtcaagcccaccgaactctgtgc and gactcttccgtccaggtactcgttgcgattgcac. Both strands of the PCR products were sequenced to confirm the target mutations, and the recombinant micro-Pgs were ligated into pET11d for bacterial expression. Recombinant micro-Pgs were purified from inclusion bodies and refolded in 55 mM Tris, pH 8.2, with 10.56 mM NaCl/0.44 mM KCl/0.055% polyethylene glycol 3350/2.2 mM MgCl2/2.2 mM CaCl2/550 mM l-arginine/1 mM reduced glutathione/0.1 mM oxidized glutathione, as described (24). The purified proteins were then dialyzed in 100 mM Tris, pH 8.0/10 mM EDTA. Active site titration of Pg, micro-Pg, SK, or SUPA was performed by using the fluorogenic substrate 4-methylumbelliferyl p-guanidinobenzoate (Sigma) to determine the active concentrations of these proteins (22). SKβswap was titrated by an indirect method (15).
Kinetic Assays. The kinetic parameters of the plasmins, microplasmins, or activator complexes were measured with a p-nitroanilide substrate H-d-valyl-l-leucyl-l-lysine-p-nitroanilide dihydrochloride (S2251), as described (13, 25), in an assay buffer (50 mM Tris·HCl/100 mM NaCl, pH 7.4). Equimolar complexes (final concentration 5 nM) were prepared by mixing plasmin or human microplasmin and SK, SUPA, or SKβswap for 10 min on ice. Plasmins and microplasmins were generated from Pg or micro-Pg by urokinase (1:30 ratio) at 37°C for 20–90 min. The reaction was initiated by addition of enzyme complexes to assay buffer containing S2251 (final concentration 50–1,500 μM), in a total volume of 100 μl in microtiter plates at 37°C. The generation of amidolytic activity (at 405 nm) was monitored at 37°C for 10 min in a microplate reader (Molecular Devices). Less than 10% of substrate was consumed during the course of the reaction. An ε1M at 405 nm of 10,000 was used for p-nitroanilide. The data were plotted as velocity vs. substrate and analyzed by hyperbolic curve fitting as described (26).
Statistical Methods. The significance of differences between sequence identities was assessed by Student's t-test. A two-tailed probability of <0.05 was considered significant.
Results and Discussion
SK produced by Streptococcus equisimilis efficiently activated human Pg but few other animal Pgs (Fig. 1B and Table 1). A similarly restricted pattern of Pg activation was seen with SUPA (or PauA), a newly discovered SK-like PA from a bovine isolate (21, 27, 28). SUPA optimally activated horse as well as dog and cow Pg but not human or other animal Pgs (Table 1 and Fig. 1C). In general, these bacterial PAs preferentially activated Pgs from the animal species that were infected by the strain of bacteria that produced them (18–20). In contrast, the animal PA urokinase activated all animal Pgs tested (see Table 1) (17).
Table 1. Reactivity of PAs with different animal Pgs.
|
PAs
|
|||||
|---|---|---|---|---|---|
| Animal Pg | Urokinase | SK | Staphylokinase | SUPA | SKβswap |
| Human | + | + | + | - | + |
| Horse | + | - | - | + | + |
| Dog | + | - | + | + | + |
| Mouse | + | - | - | - | - |
| Baboon | + | +/- | + | - | - |
| Cow | + | - | - | + | - |
| Pig | + | -/+ | - | - | - |
| Sheep | + | - | - | - | - |
| Rabbit | + | - | - | - | - |
Animal Pgs (300 nM) were activated by various Pg activators (20 nM) at 37°C, and the hydrolysis of the plasmin substrate S-2251 was monitored. Pg activators were considered to activate (+) a given Pg if they produced detectable cleavage of the S-2251 substrate within 60 min of incubation. The remaining Pg activators had trace (+/-) or no (-) detectable activity after 6 hr of incubation with Pg.
This restricted ability of SK to activate animal Pgs may arise from a failure of SK to form enzymatically active complexes with other animal plasmins or from an inability of the SK in the complex to process Pg substrate (see Fig. 1 A). SK formed an active amidolytic complex with human, horse, dog, and cow plasmin with improved affinity (decreased Km) for a small peptide paranitroanilide substrate (Table 2). A similar pattern was seen for the interactions of SUPA with cow, horse, dog, and human plasmin. That SK and SUPA formed enzymatically active complexes with other species plasmins indicated that defective complex formation per se was not responsible for species-restricted Pg activation.
Table 2. Kinetic constants for amidolysis.
|
Amidolytic parameters
|
|||
|---|---|---|---|
| Plasmin or plasmin complex | Km, μM | kcat, s-1 | kcat/Km, μM-1·s-1 |
| Human plasmin | 500 ± 90 | 14.2 ± 0.45 | 0.028 |
| SK-human plasmin | 250 ± 9 | 26.4 ± 0.6 | 0.106 |
| SKβswap-human plasmin | 125 ± 5 | 12.8 ± 0.2 | 0.102 |
| SUPA-human plasmin | 75 ± 6 | 4.9 ± 0.1 | 0.065 |
| Cow plasmin | 533 ± 15 | 58.0 ± 0.2 | 0.109 |
| SK-cow plasmin | 357 ± 21 | 18.0 ± 1.0 | 0.050 |
| SKβswap-cow plasmin | 237 ± 15 | 28.0 ± 1.0 | 0.118 |
| SUPA-cow plasmin | 125 ± 5 | 17.0 ± 1.0 | 0.136 |
| Horse plasmin | 430 ± 95 | 7.7 ± 0.2 | 0.018 |
| SK-horse plasmin | 108 ± 6 | 2.8 ± 0.3 | 0.026 |
| SKβswap-horse plasmin | 125 ± 15 | 2.9 ± 0.6 | 0.023 |
| SUPA-horse plasmin | 175 ± 14 | 4.8 ± 0.1 | 0.027 |
| Dog plasmin | 610 ± 165 | 5.5 ± 1.0 | 0.009 |
| SK-dog plasmin | 146 ± 18 | 3.5 ± 0.2 | 0.024 |
| SKβswap-dog plasmin | 196 ± 22 | 4.5 ± 0.5 | 0.023 |
| SUPA-dog plasmin | 232 ± 33 | 4.9 ± 0.6 | 0.021 |
Amidolytic experiments were carried out at 37°C in a total volume of 100 μl as described in Materials and Methods. The values represent the mean ± SE.
A crystal structure of Pg substrate bound to the SK–plasmin complex has not been obtained, but previous studies have indicated that the SK β domain plays a central role in processing Pg substrate (26, 29, 30). We examined whether species-restricted Pg activation may arise from a lack of intermolecular complementarity between the β domain and a particular Pg substrate. A SKβswap was created by swapping the β domain of SK with the β domain of SUPA. Like SUPA, but unlike SK, SKβswap gained the ability to activate horse Pg (Fig. 1D) as well as dog Pg. This gain of function provided evidence that Pg activation requires molecular complementarity between the β domains of different SKs and a particular animal Pg substrate. Similar to SK but not to SUPA, the human plasmin–SKβswap complex also remained an efficient activator of human Pg, providing indirect evidence that the other SK domains also contribute to Pg substrate binding.
Although SKβswap formed a distinct enzymatic complex with cow plasmin (Table 2), this complex was unable to process cow Pg substrate (Table 1). Thus, other factors, such as the intermolecular complementarity between SK and the plasmin moiety in the activator complex, may contribute to species-restricted Pg activation. In the crystal structure, the α and γ domains of SK have extensive molecular contacts with the catalytic domain of human plasmin (microplasmin), whereas the β domain has few interactions (16). The α and γ domains contact microplasmin via loops 5, 6, 7, 9, and 11 (Fig. 2A, loops enumerated according to chymotrypsin numbering system) (16). Human plasmin has substantial sequence identity with horse and dog plasmin at these loop sites (67% and 75%, respectively; Table 3) but weak identity with cow plasmin (25%). If molecular complementarity is a principal determinant of species-restricted Pg activation, chimerization of these loop structures of human microplasmin with the corresponding cow plasmin loop sequences may cause the resulting human microplasmin chimera to “gain function” with SUPA. The sequences of loops 5, 6, 7, 9, and 11 in human microplasmin were replaced with the corresponding bovine sequences to create a compound, human microplasmin chimera. Chimeras containing the loop 6 sequence could not be refolded to an active protein (24). The compound human micro-Pg chimera containing cow plasmin loops 5, 7, 9, 11 was efficiently activated by SUPA, although human microplasmin alone was not (Fig. 2B). At the same time, unlike human micro-Pg, the compound human micro-Pg chimera lost the ability to be activated by SK (Fig. 2C). Indeed, SK activity was sensitive to relatively minor changes in the loop sequences of human Pg. Chimerization of loop 5 of human micro-Pg with the cow loop 5 sequence reduced activity with SK compared with native human micro-Pg (Fig. 2C). Chimerization of 2 loops (5 and 7) or 3 loops (5, 7, and 11) fully eliminated activity with SK, although these chimeras retained the ability to be activated by urokinase (e.g., Fig. 2C Inset).
Fig. 2.
SK–Pg interactions. (A) A ribbon diagram illustrates the crystal structure of human Pg. The loop structures that contact the α and γ domains of SK are numbered and colored. This figure was produced by using molscript and raster3d (36, 37). (B and C) Activation of cow Pg, human micro-Pg, and chimeric micro-Pgs by SUPA (B) and SK (C). Human micro-Pg (300 nM), cow Pg (300 nM), and chimeric loop 5, 7, 9, 11 micro-Pg (300 nM) (or other chimeric micro-Pgs) were activated by SK (20 nM) or SUPA, as indicated. Pg activation was monitored as described in Fig. 1. (Inset) Activation of the loop 5, 7, 9, and 11 chimeric micro-Pg (300 nM) by urokinase (33 nM).
Table 3. Sequence similarity between human and animal plasmins at loop sites that contact the α and γ domains of SK in the SK–plasmin complex (16).
|
SK domain
|
Plasmin loop
|
Residue no.
|
Animal plasmin
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Horse | Dog | Mouse | Baboon | Bovine | Sheep | Pig | |||
| α | 5 | 606 | Glu | Glu | Glu | Glu | Glu | Asp | Asp | Glu |
| α | 7 | 644 | Arg | Arg | Arg | Asn | Gly | Gln | Arg | Glu |
| α | 7 | 645 | Lys | Ala | Ala | Arg | Ala | Ala | Ala | Ala |
| α | 11 | 713 | Tyr | Tyr | Tyr | Val | Tyr | Asn | Tyr | Tyr |
| α | 11 | 717 | Asn | Asn | Asn | Asn | Asn | Asp | Asn | Gly |
| α | 11 | 718 | Gly | Gly | Gly | Asn | Gly | Gly | Gly | Gly |
| α | 11 | 719 | Arg | Arg | Arg | Arg | Arg | Arg | Arg | Lys |
| α | 11 | 721 | Gln | Lys | Lys | Lys | Lys | Lys | Lys | Lys |
| γ | 6 | 625 | Asn | Arg | Asn | Ile | Arg | Val | Ala | His |
| γ | 6 | 626 | Leu | Leu | Leu | Arg | Leu | Arg | Arg | Leu |
| γ | 9 | 692 | Phe | Ser | Tyr | Phe | Tyr | Phe | Phe | Tyr |
| γ | 9 | 694 | Ala | Ala | Ala | Ala | Ala | Glu | Ala | Ala |
Plasmin has close structural similarity to trypsin and other chymotrypsin-like serine proteases whose loop structures mediate substrate specificity in processes such as blood clotting, complement activation, and food digestion (24, 31). Among these proteases, plasmin may be subject to unique evolutionary pressures, because it serves roles critical to the survival of both animals and their pathogenic bacteria. Animal survival requires conservation of molecular interactions between animal Pg and PAs as well as other regulatory molecules. At the same time, animal survival or resistance to bacterial infections may be promoted by competitive changes in primary structure of Pg that diminish its reactivity with bacterial PAs. If this is true, there should be significantly greater sequence variation in the loop structures (3, 5, 6, 7, 9, and 11) of plasmins that interact with SK than in the loop structures that do not (16). Among 11 different animal Pgs, there was greater sequence diversity from human plasmin in loops that contact SK (mean identity 68 ± 13%) than in loops that do not (mean identity 90 ± 5%, P < 0.01). In contrast, this sequence diversity was not seen in comparable loop structures among different animal trypsins (mean identity 85 ± 11% vs. 79 ± 18%) or among different animal chymotrypsins (mean identity 77 ± 8% vs. 84 ± 13%), providing evidence that loop structures in PA that interact with SK are differentially targeted for greater sequence variation than other comparable loop structures.
Pathogenic Streptococcus sp. may be under coevolutionary pressures to produce SKs that retain function with evolving target Pgs (20). SKs produced by pathogenic Streptococcus sp. that infect humans showed greater conservation at sites that have intermolecular contact with microplasmin in the activator complex than other positions (95% vs. 89%, P < 0.05). In contrast, SKs that activate other species Pgs showed minimal conservation at those sites when compared with SKs that activate human Pg (14% vs. 95%, P < 0.00001).
Taken together, these data suggest an evolutionary battle between animals and their bacterial pathogens for control of the PA system. Urokinase (an animal PA) has a conserved ability to cleave a wide spectrum of animal Pgs, whereas the ability of SK (a bacterial PA) has been markedly restricted. The conservation of urokinase–Pg interactions is consistent with the hypothesis that functionally important interacting partners coevolve, at sites of molecular contact, to retain biological activities that are important for survival (4–6). It is also consistent with the notion that the species-restricted Pg activation of SK arises in part from alterations in the intermolecular complementarity between SK and the plasmin moiety in the activator complex, as well as between SK and the substrate Pg. Not surprisingly, among SKs derived from different strains of Streptococcus sp. that infect humans, there is a strong conservation of residues that form molecular contacts with plasmin in the activator complex. However, among SKs from streptococci that infect other animals and activate different species Pgs, the lack of conservation of these residues is remarkable. This may reflect a coevolutionary pressure on the SK genes to retain molecular complementarity and function with specific host animal Pgs in the face of a competitive evolutionary process in animals that increases survival by altering these Pg sequences to elude subversion by these bacterial pathogens. This interplay of coevolutionary and competitive evolutionary pressures is probably not unique to Streptococcus sp. but may also occur when other pathogenic organisms (e.g., HIV, Staphylococcus) produce molecules that seek to exploit other biological pathways (e.g., chemokine receptors and coagulation proteins) in animals to enhance their survival in the host (32–35).
Acknowledgments
We are grateful to Drs. Lizbeth Hedstrom, Janos Polgar, and Brian Robinson for helpful suggestions and to Aiilyan Houng for technical contributions. This work was supported in part by National Institutes of Health Grant HL58496 (to G.L.R.), an American Heart Association Fellowship (to I.Y.S.), a Minority Graduate Research Assistant Award (HL-58496, to R.B.T.), and a Howard Hughes Medical Institute Fellowship (to R.B.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Pg, plasminogen; PAs, Pg activators; SUPA, Streptococcus uberis PA; SK, streptokinase; SKβswap, SK α-SUPA β-SK γ chimera.
References
- 1.Parry, M. A., Zhang, X. C. & Bode, I. (2000) Trends Biochem. Sci. 25, 53–59. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet, P. & Collen, D. (1995) Thromb. Haemostasis 74, 429–436. [PubMed] [Google Scholar]
- 3.Robbins, K. C., Summaria, L., Hsieh, B. & Shah, R. J. (1967) J. Biol. Chem. 242, 2333–2342. [PubMed] [Google Scholar]
- 4.Goh, C. S., Bogan, A. A., Joachimiak, M., Walther, D. & Cohen, F. E. (2000) J. Mol. Biol. 299, 283–293. [DOI] [PubMed] [Google Scholar]
- 5.Pazos, F., Helmer-Citterich, M., Ausiello, G. & Valencia, A. (1997) J. Mol. Biol. 271, 511–523. [DOI] [PubMed] [Google Scholar]
- 6.Giuliani, A. & Tomasi, M. (2002) Proteins 46, 171–176. [DOI] [PubMed] [Google Scholar]
- 7.Hornef, M. W., Wick, M. J., Rhen, M. & Normark, S. (2002) Nat. Immunol. 3, 1033–1040. [DOI] [PubMed] [Google Scholar]
- 8.Tillet, W. S. & Garner, R. L. (1933) J. Exp. Med. 58, 485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lack, C. H. (1948) Nature 161, 559–560. [DOI] [PubMed] [Google Scholar]
- 10.Lahteenmaki, K., Kuusela, P. & Korhonen, T. K. (2001) FEMS Microbiol. Rev. 25, 531–552. [DOI] [PubMed] [Google Scholar]
- 11.Li, Z., Ploplis, V. A., French, E. L. & Boyle, M. D. (1999) J. Infect. Dis. 179, 907–914. [DOI] [PubMed] [Google Scholar]
- 12.Sodeinde, O. A., Subrahmanyam, Y. V., Stark, K., Quan, T., Bao, Y. & Goguen, J. D. (1992) Science 258, 1004–1007. [DOI] [PubMed] [Google Scholar]
- 13.Reed, G. L., Houng, A. K., Liu, L., Parhami-Seren, B., Matsueda, L. H., Wang, S. & Hedstrom, L. (1999) Proc. Natl. Acad. Sci. USA 96, 8879–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cederholm-Williams, S. A., De Cock, F., Lijnen, H. R. & Collen, D. (1979) Eur. J. Biochem. 100, 125–132. [DOI] [PubMed] [Google Scholar]
- 15.Gladysheva, I. P., Sazonova, I. Y., Chowdhry, S. A., Liu, L., Turner, R. B. & Reed, G. L. (2002) J. Biol. Chem. 277, 26846–26851. [DOI] [PubMed] [Google Scholar]
- 16.Wang, X., Lin, X., Loy, J. A., Tang, J. & Zhang, X. C. (1998) Science 281, 1662–1665. [DOI] [PubMed] [Google Scholar]
- 17.Wohl, R. C., Sinio, L., Summaria, L. & Robbins, K. C. (1983) Biochim. Biophys. Acta 745, 20–31. [DOI] [PubMed] [Google Scholar]
- 18.McCoy, H. E., Broder, C. C. & Lottenberg, R. (1991) J. Infect. Dis. 164, 515–521. [DOI] [PubMed] [Google Scholar]
- 19.Leigh, J. A. (1993) FEMS Microbiol. Lett. 114, 67–71. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder, B., Boyle, M. D., Sheerin, B. R., Asbury, A. C. & Lottenberg, R. (1999) Infect. Immun. 67, 6487–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sazonova, I. Y., Houng, A. K., Chowdhry, S. A., Robinson, B. R., Hedstrom, L. & Reed, G. L. (2001) J. Biol. Chem. 276, 12609–12613. [DOI] [PubMed] [Google Scholar]
- 22.Reed, G. L., Lin, L. F., Parhami, S. B. & Kussie, P. (1995) Biochemistry 34, 10266–10271. [DOI] [PubMed] [Google Scholar]
- 23.Wang, S., Reed, G. L. & Hedstrom, L. (2000) Eur. J. Biochem. 267, 3994–4001. [DOI] [PubMed] [Google Scholar]
- 24.Turner, R. B., Liu, L., Sazonova, I. Y. & Reed, G. L. (2002) J. Biol. Chem. 277, 33068–33074. [DOI] [PubMed] [Google Scholar]
- 25.Wohl, R. C., Summaria, L. & Robbins, K. C. (1980) J. Biol. Chem. 255, 2005–2013. [PubMed] [Google Scholar]
- 26.Lin, L. F., Oeun, S., Houng, A. & Reed, G. L. (1996) Biochemistry 35, 16879–16885. [DOI] [PubMed] [Google Scholar]
- 27.Johnsen, L. B., Poulsen, K., Kilian, M. & Petersen, T. E. (1999) Infect. Immun. 67, 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosey, E. L., Lincoln, R. A., Ward, P. N., Yancey, R. J., Jr., & Leigh, J. A. (1999) FEMS Microbiol. Lett. 178, 27–33. [DOI] [PubMed] [Google Scholar]
- 29.Nihalani, D., Raghava, G. P. & Sahni, G. (1997) Protein Sci. 6, 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, X., Tang, J., Hunter, B. & Zhang, X. C. (1999) FEBS Lett. 459, 85–89. [DOI] [PubMed] [Google Scholar]
- 31.Perona, J. J. & Craik, C. S. (1997) J. Biol. Chem. 272, 29987–29990. [DOI] [PubMed] [Google Scholar]
- 32.Proudfoot, A. E., Power, C. A. & Wells, T. N. (2000) Immunol. Rev. 177, 246–256. [DOI] [PubMed] [Google Scholar]
- 33.Bates, P. (1996) Cell 86, 1–3. [DOI] [PubMed] [Google Scholar]
- 34.Tager, M. & Drummond, M. C. (1965) Ann. N.Y. Acad. Sci. 128, 92–111. [DOI] [PubMed] [Google Scholar]
- 35.Parry, M. A., Fernandez-Catalan, C., Bergner, A., Huber, R., Hopfner, K. P., Schlott, B., Guhrs, K. H. & Bode, W. (1998) Nat. Struct. Biol. 5, 917–923. [DOI] [PubMed] [Google Scholar]
- 36.Kraulis, P. J. (1991) J. Appl. Crystallogr. 24, 946–950. [Google Scholar]
- 37.Bacon, D. J. & Anderson, W. F. (1988) J. Mol. Graphics 6, 219–220. [Google Scholar]