Abstract
Adhering cells actively probe the mechanical properties of their environment and use the resulting information to position and orient themselves. We show that a large body of experimental observations can be consistently explained from one unifying principle, namely that cells strengthen contacts and cytoskeleton in the direction of large effective stiffness. Using linear elasticity theory to model the extracellular environment, we calculate optimal cell organization for several situations of interest and find excellent agreement with experiments for fibroblasts, both on elastic substrates and in collagen gels: cells orient in the direction of external tensile strain; they orient parallel and normal to free and clamped surfaces, respectively; and they interact elastically to form strings. Our method can be applied for rational design of tissue equivalents. Moreover, our results indicate that the concept of contact guidance has to be reevaluated. We also suggest that cell–matrix contacts are up-regulated by large effective stiffness in the environment because, in this way, build-up of force is more efficient.
The mechanical activity of adherent cells usually is attributed to their physiological function. For example, fibroblasts are believed to maintain the structural integrity of connective tissue and to participate in wound healing by actively pulling on their environment. During recent years, it has become clear that there is another important role for mechanical activity of adherent cells: by pulling on their environment, cells can actively sense its mechanical properties and react to it in a specific way (1–3). Harris et al. (4) observed surprisingly large tension fields for fibroblasts on elastic substrates, which induce mechanical activity of other cells, even when located at considerable distance. When plated on elastic substrates of increased rigidity, many cell types show increased spreading and better developed stress fibers and focal adhesions (5). Fibroblasts on elastic substrates orient in the direction of tensile strain (6) and locomote in favor of regions of larger rigidity or tensile strain (7). The same response has been reported for vascular smooth muscle cells on rigidity gradients (8). Similar observations have been reported numerous times also for tissue cells in hydrogels. For fibroblasts in collagen gels, Bell et al. (9) not only found that traction considerably contracts the gel, but also reported orientational effects: cells align along the direction of pull between fixed points and parallel to free surfaces. When a collagen gel is stretched uniaxially, cells orient in the direction of principal strain (10). Moreover, cells align in a nose-to-tail configuration, thus forming strings running in parallel to the direction of external strain. If a collagen gel is cut perpendicular to the direction of tensile strain and if cells are present in sufficient numbers, they round up and reorient parallel to the free surface introduced (11).
The response of adherent animal cells to mechanical input has evolved in the physiological context of a multicellular organism and plays a crucial role in development, tissue maintenance, angiogenesis, wound contraction, inflammation, and metastasis. Recently, there has been a large experimental effort to understand its molecular basis. A growing body of evidence suggests that focal adhesions based on transmembrane receptors from the integrin family act as mechanosensors that directly feed into cellular regulation (12). In particular, the application of external force to focal adhesions leads to their structural reinforcement and strong signaling activity (13–15), and internally generated force correlates with the state of aggregation of mature focal adhesions (16, 17). The exact mechanism of the mechanosensor at focal adhesions is still unknown, although structural reorganization of the whole aggregate or conformational changes of specific molecules are likely candidates. Although focal adhesions are characteristic for cells cultured on flat and rigid substrates, cells in a soft environment develop similar cell–matrix contacts that presumably have the same mechanosensory function (18).
As a result of active mechanosensing at cell–matrix contacts, cells remodel their contacts and cytoskeleton. In particular, they might change position and become oriented in a certain direction, depending on the mechanical properties of their environment. Although cellular behavior in principle results from very complex regulatory processes, here we show that the typical cellular reaction to mechanical input seems to be a simple preference for large effective stiffness: starting from this principle, we are able to explain many experimental findings that have been reported for the behavior of adherent cells both on elastic substrates and in hydrogels. To make these predictions, we have to calculate how stress and strain propagates in the extracellular environment. For this purpose, we model it with linear elasticity theory and solve the elastic equations for different geometries and boundary conditions of interest. We then calculate the position and orientation in which the cell senses maximal effective stiffness in its local environment. Predicting cell organization in a soft medium not only contributes to a better understanding of many physiological situations, but also is of large practical value for application in tissue engineering, e.g., when culturing fibroblasts in collagen gels.
Theory
Optimization Principle for Single Contact. Motivated mainly by recent experiments with elastic substrates (5, 7, 8), we suggest that an adherent cell positions and orients itself in such a way that it senses maximal effective stiffness in its environment. In these experiments, the most relevant input for cellular decisionmaking is local elasticity of the surrounding environment. Thus we first have to calculate how stress and strain in the medium are propagated toward the cell. These calculations are in general very complicated and will be presented below for different situations of interest. To keep our calculations feasible, we assume that the extracellular environment is described by isotropic linear elasticity theory. This assumption holds true for most synthetic elastic substrates and might be reasonable for hydrogels. Hence, there are two elastic moduli, the Young modulus E (which describes rigidity) and the Poisson ratio ν (which describes the relative weight of compression and shear modes). In practice, E will be on the order of kilopascals, which is a typical physiological value for tissue stiffness. In most situations, ν is expected to be close to one-half (the value for an incompressible medium), but other values might be realized in future applications.
We then ask in which way the cell will organize itself if it probes its
local environment by actively pulling on it. In particular, we aim to define a
quantity that describes the kind of information that the cell can extract from
its soft environment with the help of its contractile machinery. We first
consider a single cell–matrix contact and suggest that an appropriate
choice is the work W that the cell has to invest into the surrounding
elastic medium to build up some force 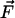 at the contact position
at the contact position
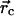 . As we will show
now, the quantity W can be used to describe the effects of increased
rigidity E and prestrain in the elastic environment on an equal
basis. Therefore W is a measure for the effective stiffness of the
elastic environment as probed through a single contact.
. As we will show
now, the quantity W can be used to describe the effects of increased
rigidity E and prestrain in the elastic environment on an equal
basis. Therefore W is a measure for the effective stiffness of the
elastic environment as probed through a single contact.
In the absence of prestrain, the work W invested into the environment is
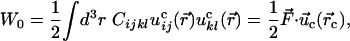 |
[1] |
where summation over repeated indices is implied. Here
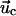 is the
displacement caused by the cell,
is the
displacement caused by the cell,
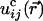 is the corresponding strain tensor, and Cijkl is
the elastic constant tensor based on E and ν. The volume integral
runs over the whole space filled with extracellular material, and its
conversion into a local expression requires partial integration and use of the
mechanical equilibrium conditions (details of our calculations will be
published elsewhere). Formally, the self-energy of a given contact diverges
for a point force, but this divergence can easily be removed by assuming
distributed force. Because displacement decreases with increasing rigidity
(uij ∼ 1/E), the cell has to invest
less work W0 to achieve a certain force
is the corresponding strain tensor, and Cijkl is
the elastic constant tensor based on E and ν. The volume integral
runs over the whole space filled with extracellular material, and its
conversion into a local expression requires partial integration and use of the
mechanical equilibrium conditions (details of our calculations will be
published elsewhere). Formally, the self-energy of a given contact diverges
for a point force, but this divergence can easily be removed by assuming
distributed force. Because displacement decreases with increasing rigidity
(uij ∼ 1/E), the cell has to invest
less work W0 to achieve a certain force
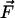 when rigidity E increases.
Hence, the cell senses maximal stiffness at the contact when
W0 is minimal.
when rigidity E increases.
Hence, the cell senses maximal stiffness at the contact when
W0 is minimal.
In a homogeneous medium, the elastic constants do not change and
W0 is a constant. However, the work W needed to
build up some force 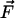 at the contact
position
at the contact
position 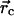 can vary
because of the presence of prestrain. The corresponding contribution to
W is
can vary
because of the presence of prestrain. The corresponding contribution to
W is
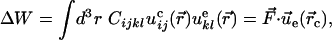 |
[2] |
where 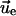 is the
displacement caused by the external strain and
is the
displacement caused by the external strain and
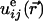 is the corresponding strain tensor. Because a negative ΔW
reduces the cellular work W = W0 +
ΔW, as does a larger rigidity E, it represents an
effective stiffening of the environment (strain-stiffening).
Correspondingly, a positive ΔW represents an effective
softening with respect to the unstrained medium. Therefore the quantity
W allows us to characterize the local elastic input available to an
actively mechanosensing cell within the unifying concept of effective
stiffness, independent of its physical origin, which might be rigidity or
prestrain. In the following, we will identify optimal cell position and
orientation with the specific force pattern that minimizes the quantity
W. In the sense described here, this corresponds to a cellular
preference for maximal effective stiffness in its local elastic
environment.
is the corresponding strain tensor. Because a negative ΔW
reduces the cellular work W = W0 +
ΔW, as does a larger rigidity E, it represents an
effective stiffening of the environment (strain-stiffening).
Correspondingly, a positive ΔW represents an effective
softening with respect to the unstrained medium. Therefore the quantity
W allows us to characterize the local elastic input available to an
actively mechanosensing cell within the unifying concept of effective
stiffness, independent of its physical origin, which might be rigidity or
prestrain. In the following, we will identify optimal cell position and
orientation with the specific force pattern that minimizes the quantity
W. In the sense described here, this corresponds to a cellular
preference for maximal effective stiffness in its local elastic
environment.
It is important to note that conceptually the principle suggested here does not imply that the cell actually minimizes the work W invested into its soft environment. Instead we suggest here that calculating the quantity W for different situations of interest is an appropriate measure for the kind of information a cell can extract from its elastic environment through active mechanosensing. The real justification of our model will be its success in explaining a large body of experimental data (see Results). Nevertheless, below we will also present a potential mechanism for the cellular preference for effective stiffness, which in fact uses the quantity W not as a characterization of the external environment but as a relevant quantity for some internal mechanism.
Optimization Principle for Cellular Force Pattern. Different
contacts are coupled through the actin cytoskeleton in such a way that overall
force balance is ensured. We account for this constraint by considering only
pairs of opposing forces. In elasticity theory, such a pinching force pattern
is known as an anisotropic force contraction dipole, that is the
tensor Pij =
Pninj, where
P is the dipole strength, the product of force magnitude and force
separation, and 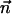 is its orientation
(19–21).
Typical cellular dipoles have been measured to be of the order of P
≈ –10–11 J (this corresponds to two
forces of 200 nN each, separated by a distance of 60 μm)
(22). The effect of external
strain on the work required to build up the force dipole
Pij at the cell position
is its orientation
(19–21).
Typical cellular dipoles have been measured to be of the order of P
≈ –10–11 J (this corresponds to two
forces of 200 nN each, separated by a distance of 60 μm)
(22). The effect of external
strain on the work required to build up the force dipole
Pij at the cell position
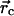 can be written as
can be written as
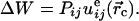 |
[3] |
As in the case of a single contact, optimal cell organization can be
identified with the specific force pattern that minimizes ΔW.
It follows directly from Eq. 3 that because of the contractile activity
of the cell (P < 0), tensile strain
(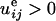 ) will always be favorable
(negative ΔW). In contrast to compressive strain
(
) will always be favorable
(negative ΔW). In contrast to compressive strain
(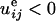 ), it corresponds to an
effective increase in stiffness. Note that in contrast to position,
orientation, and sign, the magnitude |P| of the
cellular force pattern does not matter in the model presented here. This
reflects the fact that here we aim to characterize the mechanical properties
of the extracellular environment sensed by the cell, rather than the process
of active mechanosensing itself.
), it corresponds to an
effective increase in stiffness. Note that in contrast to position,
orientation, and sign, the magnitude |P| of the
cellular force pattern does not matter in the model presented here. This
reflects the fact that here we aim to characterize the mechanical properties
of the extracellular environment sensed by the cell, rather than the process
of active mechanosensing itself.
Possible Origin of Optimization Principle. Our modeling starts from the phenomenological observation that cells seem to prefer maximal effective stiffness in their environment. Although it can be justified by its large success in explaining experimental observations (see Results), we also want to suggest a possible mechanism for our main assumption. For this purpose, we use a simple one-dimensional analogue. Consider the extracellular environment to act like a linear spring with spring constant K, on which the cell is pulling through a single cell–matrix contact. Recent experiments on focal adhesions (15) suggest that up-regulation of contact growth is related to reaching a certain threshold in force F, although the details of how force affects regulation are just beginning to emerge (12). To build up sufficiently large force F, the cell has to invest energy W = F2/2K into the spring. Thus, the stiffer the spring (the larger K), the less work is needed and the more efficient the build-up of force will be. An equivalent viewpoint is to assume that the cell invests the power L into stretching the spring. Then it takes the time t = F2/2KL to reach the force F. Therefore, a specific contact will grow faster than the other contacts if it encounters a larger stiffness K. In principle, the cellular program could also be geared toward achieving a certain displacement of the surrounding material, which would result in a preference for effective softness of the environment. However, this scenario would imply the existence of some additional mechanism for outside-in signaling. It is more realistic to consider that force activates the cellular response through a certain displacement of elastic components located inside the cell. Because internal displacement and force are expected to be linearly related through another (internal) spring constant, one arrives at the same result.
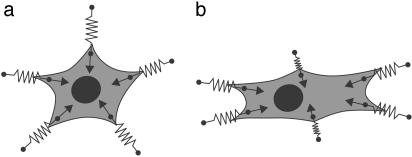
Adhering cells probe the mechanical properties of their environment by pulling at many cell–matrix contacts simultaneously (Fig. 1). At each newly formed contact the cell is expected to pull with a similar investment of resources (e.g., actin, myosin, or ATP). However, each contact encounters a different elastic environment, each of which can be represented by a different spring constant. In an isotropic situation (Fig. 1a), all spring constants are equal, the contacts have similar growth behavior, and there is no reason for a cell to orient. Experimentally, the cell adopts a round or stellate morphology, depending on the number of cell–matrix contacts. In an anisotropic situation (Fig. 1b), build-up of force is more efficient in one specific direction and contacts in this direction will eventually outgrow the other ones. Here, the anisotropic elastic properties of the medium provide an orientational clue for the cell, which orients along the direction of maximal effective stiffness. Depending on, e.g., the presence of motility factors, this orientation response might be followed by cell locomotion.
Fig. 1.
An adherent cell actively pulls on its soft environment through cell–matrix contacts. Experimentally, one finds that cells orient themselves in the direction of maximal stiffness of the environment. In this cartoon, we present one possible mechanism by which active mechanosensing in an elastically anisotropic medium might lead to cell orientation. The local elastic environment is represented by linear springs with different spring constants K, as indicated by differently sized springs. For up-regulation of a contact, the cell has to invest the work F2/2K. Therefore, up-regulation is more efficient for larger K. (a) In an isotropic environment, all spring constants are the same, growth at different contacts is similar, and the cell does not orient. (b) If spring constants are largest in one specific direction, the corresponding contacts outgrow the others and the cell orients in the direction of maximal stiffness of the environment. In this paper, we use the cellular preference for large effective stiffness and modeling of the extracellular environment by linear elasticity theory to predict cell positioning and orientation in soft media.
Results
Homogeneous External Strain. We first consider a cell interacting with homogeneous external strain, either on the top surface of a rectangular slab of elastic material (elastic substrate) or inside an infinite elastic material (hydrogel). In both cases, the equations of three-dimensional isotropic elasticity give
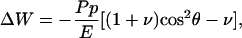 |
[4] |
where θ is the orientation angle relative to the direction of the externally applied tensile stress p < 0. Optimal cell orientation corresponds to minimal ΔW, which is achieved for θ = 0, irrespective of the Poisson ratio ν. Thus the cell orients preferentially with the direction of stretch. This behavior is indeed observed experimentally, for fibroblasts both on elastic substrates (6) and in collagen gels (9, 10). Because ΔW decreases with increasing rigidity E, the elastic effects discussed here will be observed only in a soft environment, namely with rigidity E around kilopascals, which is a typical physiological value for tissue stiffness. For stiffer substrates the variations in ΔW for different contact positions might become too small to induce an orientation response.
Boundaries. In a physiological context, cells are often close to boundaries, such as the surface of a tissue or organ. In the presence of cell traction, boundaries alter the strain with respect to a homogeneous infinite medium by a boundary-induced strain (image strain), which has an effect similar to that of external strain. In this way, cells can actively sense not only the presence of a close-by surface, but also its shape and boundary conditions. To predict the effect of boundaries on cell organization, we study a semiinfinite space with a planar surface, for which the elastic equations can be solved exactly (23). The details of the boundary conditions in a physiological context can be very complicated. Here we address two fundamental reference cases, namely free and clamped boundary conditions, for which normal stress and displacement, respectively, vanish at the surface. Consider a force dipole that is a distance d away from the planar surface and has an angle of orientation θ relative to the surface normal. We find
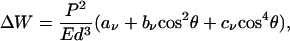 |
[5] |
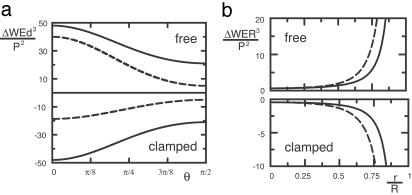
where the coefficients aν, bν, and cν are complicated functions of ν. ΔW scales quadratically with P because the image strain scales linearly with P (in other words, the force dipole interacts with its images). For free and clamped surfaces, the coefficients are positive and negative, respectively, irrespective of ν. Therefore, the optimal configurations (minimal ΔW) are parallel (θ = π/2) and perpendicular (θ = 0) for free and clamped boundaries, respectively, as plotted in Fig. 2a. A schematic representation (Fig. 3 a and b) provides a simple interpretation: for clamped (free) boundary conditions, the cell senses maximal stiffness perpendicular (parallel) to the surface. One may think of a clamped (free) surface as the interface between the medium and an imaginary medium of infinite (vanishing) rigidity, which effectively rigidifies (softens) the medium toward the boundary. In general, we find that free and clamped boundary conditions always have opposite effects, albeit with one essential difference: for clamped boundaries, mechanical activity of cells is favored and cells can amplify this effect by adjusting orientation. For free boundaries, mechanical activity of cells is disfavored and the orientation response is an aversion response.
Fig. 2.
Adjusting cell position and orientation in such a way that the cell sensing maximal effective stiffness in its environment is equivalent to minimizing the quantity W, the amount of work the cell invests into the elastic surroundings in the presence of external strain. In the presence of mechanical activity, sample boundaries induce external strain that can result in different cell organization. (a) ΔW for a cell with dipole strength P that is a distance d away from the surface of an elastic halfspace with rigidity E, plotted in units of P2/Ed3 as a function of angle θ between cell orientation and surface normal (rescaled by 256π). Solid and dashed lines correspond to Poisson ratios ν = ½ and ν = 0, respectively. Irrespective of ν, the optimal orientations (minimal ΔW) are perpendicular (θ = 0) and parallel (θ = π/2) to the surface for clamped and free boundaries, respectively. Because |ΔW| increases if d decreases, the overall mechanical activity of a cell increases toward a clamped surface (ΔW < 0) but decreases toward a free surface (ΔW > 0). (b) ΔW for a cell in an elastic sphere of radius R, plotted in units of P/ER3 as a function of distance r to the sphere center in units of R for ν = ⅓ (rescaled by 15/8). Solid and dashed lines are parallel (θ = π/2) and perpendicular (θ = 0) orientations, respectively (all other orientations yield curves that lie in between the ones shown). As in an elastic halfspace, parallel and perpendicular orientations are favored (minimal ΔW) for free and clamped boundaries, respectively. For clamped boundaries, mechanical activity is favored (smaller ΔW) toward the surface. For free boundaries, mechanical activity is disfavored (larger ΔW) toward the surface.
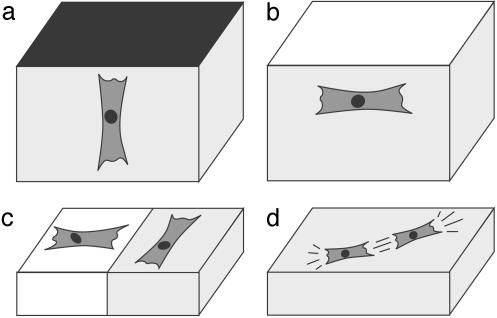
Fig. 3.
Predicted cell orientation in a hydrogel close to a surface (a and b) and on elastic substrates (c and d). (a) Cells prefer the direction of maximal effective stiffness. Thus, they orient perpendicular to a clamped surface. (b) For a free surface, this direction is parallel to the surface. (c) Cells close to a boundary between soft (left) and rigid (right) regions prefer analogous orientations as cells close to clamped and free surfaces in a hydrogel, respectively. (d) Cells interact elastically to form strings, because, in nose-to-tail alignment, the mechanical activity of one cell triggers the activity of the other cell, thereby forming a positive feedback loop.
Experimentally, it is well known that mechanical activity of cells increases for clamped boundary conditions (24). The predicted orientation effects close to boundaries have been observed numerous times, e.g., the parallel orientation of cells close to free surfaces (9). Our model predicts the same orientation effects for an elastic substrate with two regions of different rigidities (Fig. 3c): cells on the soft and stiff sides of the boundary orient perpendicular and parallel to it, respectively. Indeed, fibroblasts migrating from a soft to a stiff region keep their perpendicular orientation and cross over to the stiff side, whereas fibroblasts migrating from a stiff to a soft region do not cross the boundary, but turn by 90° and move parallel to the boundary (7).
Finite-Sized Sample. As an example for a finite-sized sample, we consider an elastic sphere with radius R. The elastic equations can be solved exactly by using an expansion in terms of vector spherical harmonics (25). We find
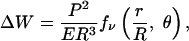 |
[6] |
where r denotes distance to the sphere center, θ is the orientation with respect to the radial direction, and fν is an infinite sum over all angular momenta, which does not change qualitatively as ν is varied. With regard to orientation, we find the same results as for the elastic halfspace (compare Fig. 2b): cells will orient parallel (perpendicular) to free (clamped) surfaces, respectively. We also find a similar result for the effect of distance to the surface: for free (clamped) boundary conditions, a small (large) distance to the sphere center is more favorable, because the surface favors (disfavors) mechanical activity. The new aspect here is the role of sphere radius R: because |ΔW| increases when sphere radius R decreases, one can effectively rigidify (soften) a material with clamped (free) boundaries by reducing system size. Our predictions could be tested by using, e.g., fibroblast-populated collagen microspheres, an assay which has been introduced to study compaction of tissue equivalents at high cell density (26). Because here we are mainly concerned with single-cell effects, we suggest to modify this assay in such a way as to monitor the organization of isolated cells close to the sphere surface at low cell density and as a function of varying sphere radius.
Cooperative Effects. Up to now we have been discussing single-cell effects; we now turn to cooperative effects. In particular, we now consider the case that external strain is caused by the traction of other cells, which amounts to an elastic interaction of cells. Even if all cells initially have isotropic force patterns, they will sense anisotropic strain fields and start to orient themselves. For the simplest case of two cells, we find
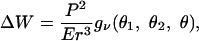 |
[7] |
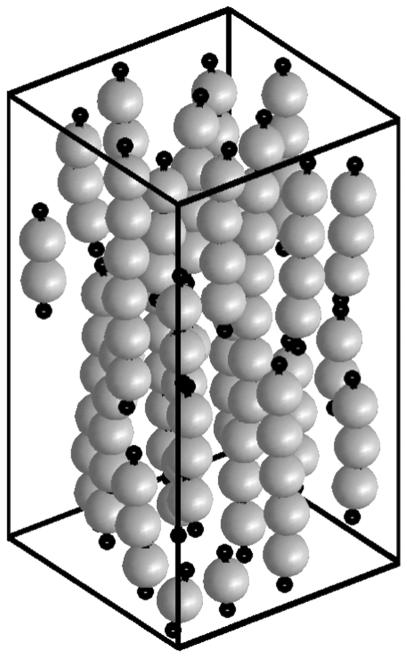
where r is the distance between the force dipoles and gν is a complicated function of ν and the three orientational degrees of freedom. Our calculation shows that ΔW has a pronounced minimum for completely aligned dipoles, independent of ν (Fig. 3d). In this configuration, both cells sense maximal effective stiffness, because maximal strain stiffening occurs along the axis of contraction. This finding suggests that a common pattern for the organization of elastically interacting cells will be the formation of strings of cells. They might close into rings, so that each cell can be fully activated by its two neighbors. We used Monte Carlo simulations to obtain a typical configuration of elastically interacting cells in an external strain field (Fig. 4). The temperature of the Monte Carlo simulation represents the stochastic nature of the orientation response. We find strings of cells aligned in parallel with the external strain, exactly as observed experimentally (10).
Fig. 4.
Monte Carlo simulations of elastically interacting cells in an external strain field. The temperature used in the simulation represents the stochastic element of the process of cell organization. Without external strain, cells form strings. In its presence, strings align in parallel.
It is important to note that there is a positive feedback for cell alignment: the more cells orient in one direction, the stronger becomes the input for other cells to adopt the same orientation. For example, it has been reported that when boundary condition are changed from clamped to free by cutting the collagen gel, fibroblasts show the predicted reorientation by 90° only when sufficiently many cells are present (11). In fact, our calculations and simulations show that cells can orient in parallel even with respect to clamped boundaries, if there are sufficiently many cells such that the direct elastic interaction between cells dominates the single-cell response of perpendicular orientation. In practice, the single-cell response might also be disturbed because elastic signals could be screened by traction of randomly oriented cells. Indeed, such an effect has been reported for experiments with elastic substrates (7).
Discussion
It has long been known, especially in the medical and bioengineering communities, that cell organization in soft media is strongly influenced by the mechanical properties of the environment. Here we presented a model that is able to explain numerous experimental observations that have been reported for organization of cells (especially fibroblasts) both on elastic substrates and in hydrogels. The excellent agreement of our results with experiments suggests that cell organization can be predicted from local mechanical properties that the cell actively senses in its environment. In fact, the only property of cellular regulation that enters our model is the assumption that cells locally prefer large effective stiffness. Otherwise our modeling focuses on the elastic properties of the extracellular environment.
Modeling the soft environment of cells as an isotropic elastic medium is certainly a good assumption for elastic substrates. The situation is more complicated for hydrogels, in particular because they might not behave elastically and because they feature fiber degrees of freedom. Cell organization in gels is often explained by contact guidance, the alignment of cells along topographic features such as collagen fibers. Since fibers can become aligned because of cell traction, contact guidance provides a long-ranged and persistent mechanism for cellular self-organization in tissue equivalents (27). This process has been modeled before. In the theory of ref. 27, flux equations for cellular and matrix densities are combined with mechanical equations that include cells as centers of isotropic contraction. This might be a good model for chondrocytes, which tend to show a spherical morphology. The anisotropic biphasic theory (ABT) from ref. 28 aims at cells such as fibroblasts and smooth muscle cells, whose typical morphology in tissue equivalents is bipolar. ABT introduces a cell orientation tensor, which is coupled to a fiber orientation tensor, because cells are assumed to react foremost to fiber degrees of freedom. In our model, the force dipole tensor represents cell orientation, as does the cell orientation tensor in ABT, but it is coupled to elastic degrees of freedom, because cells are assumed to react foremost to large effective stiffness.
Because models for contact guidance in tissue equivalents focus on fiber degrees of freedom and high cell densities, they do not explain the single-cell responses observed on elastic substrates, where contact guidance usually is ruled out (6, 7). The large predictive power of our model for elastic substrate experiments suggests that active mechanosensing by single cells might also be involved with cell organization in hydrogels. However, for the collagen assay from ref. 29 it has been shown that as a result of external strain, fibers become rearranged and stress relaxes toward zero. In a matrix that cannot support any stress, our elastic considerations do not apply and contact guidance through formerly aligned fibers might be the only relevant clue for cell organization (29). However, it is important to note that in our model, stress is actively generated by cells and thus needs to be supported only over time scales in which the cell actively senses the mechanical properties of its environment. In particular, if fiber alignment has resulted in some anisotropic mechanical environment, the cell might sense the anisotropic mechanical properties of the matrix and orient itself correspondingly. This orientation might explain why cells have been found to align to a greater extent with respect to external strain than the surrounding collagen fibrils (29) and why our modeling is also successful for hydrogels. In general, further experiments are needed to clarify the relative importance of topographic versus mechanical clues for cell organization in hydrogels, and further modeling is needed to account for the mechanical (in particular, viscoelastic) properties of hydrogels.
We also point out that contact guidance is a bidirectional clue and provides only guidance, in contrast to external elastic strain, which provides taxis. In our model, taxis is reflected by the position dependence of ΔW. For example, our theory predicts not only that cells prefer to orient parallel to free boundaries but also that cells prefer to move away from them. Moreover, a simple preference for cell alignment along fibers does not predict what cells do if they encounter a fiber junction in the gel. Although we are not concerned with cell locomotion here, our modeling suggests that cells prefer the fiber under largest tension, exactly as has been observed experimentally for neutrophils migrating in human amnion (30).
In recent years, the regulated response to mechanical input by single cells has been studied experimentally in greater detail. There is a growing body of evidence now that integrin-based cell–matrix contacts act as local mechanosensors that channel mechanical information about the environment directly into cellular decision-making. Although this does not concern our modeling directly, here we suggested that the up-regulation of growth of cell–matrix contacts in a stiff environment might result from the fact that it is triggered by a threshold in force, whose build-up is more efficient for larger stiffness. An equivalent viewpoint is that growth of cell–matrix contacts is faster on stiffer substrates. As an experimental test for this hypothesis, we suggest correlation studies for growth of cell–matrix contacts and cellular organization, especially close to sample boundaries, where cells can amplify the mechanical input provided by boundary-induced strain through active mechanosensing. Quantitative data about growth behavior of cell–matrix contact will allow us to further refine our model in a more quantitative way, possibly also including modeling of cellular features such as morphology and force pattern, which are not the focus of this work. It is important to note that our model suggests completely different behavior for cells than one would expect for physically inert particles interacting with a soft matrix (such as mobile inclusions in a metal). Although the expression in Eq. 3 is similar to the interaction potential for the physical system of force dipoles in an external strain field, it has the opposite sign, because in the physical system, one has to minimize the composite energy of defect and medium (19, 20). The physical potential has been used before to model elastic interactions of cells without any regulatory response and has been shown to lead to aggregation behavior similar to that of electric quadrupoles (21), whereas the model introduced here leads to aggregation behavior similar to that of electric dipoles (31).
Because elastic effects are long-ranged and propagate quickly, they provide an appealing mechanism for signal transduction for mechanically active cells in soft media. However, they are also unspecific and cells might not be able to distinguish between different sources. On the other hand, additional information channels, such as soluble ligands, will certainly supplement elastic signals. Moreover, cells in highly differentiated organisms are likely to interpret mechanical signals only in their own physiological context, which is more restricted than for cells in an arbitrary environment.
In summary, we have presented an optimization principle in linear elasticity theory that allows us to predict cell organization in soft media in excellent agreement with a large body of experiments. Moreover, we have suggested a mechanism that links the cellular preference for large effective stiffness to growth of cell–matrix contacts. Our modeling results in many interesting predictions that now can be checked experimentally. In the future, our model might be extended to high cell densities and strong cooperative effects, which are characteristic for tissue equivalents. We expect that then it can be used for rational design in tissue engineering. For example, using numerical (finite-element) methods, one can use it to optimize protocols for the design of tissue equivalents for implants with regard to geometry and boundary conditions.
Acknowledgments
We thank Martin Bastmeyer, Benjamin Geiger, Dirk Lehnert, Rudolf Merkel, and Samuel Safran for critical reading of an earlier version of the manuscript. We also thank the anonymous referees for helpful suggestions. This work was supported by the Emmy Noether Program of the German Science Foundation.
References
- 1.Galbraith, C. G. & Sheetz, M. (1998) Curr. Opin. Cell Biol. 10, 566–571. [DOI] [PubMed] [Google Scholar]
- 2.Huang, S. & Ingber, D. E. (1999) Nat. Cell Biol. 1, E131–E138. [DOI] [PubMed] [Google Scholar]
- 3.Geiger, B. & Bershadsky, A. (2002) Cell 110, 139–142. [DOI] [PubMed] [Google Scholar]
- 4.Harris, A. K., Wild, P. & Stopak, D. (1980) Science 208, 177–179. [DOI] [PubMed] [Google Scholar]
- 5.Pelham, R. J. & Wang, Y.-L. (1997) Proc. Natl. Acad. Sci. USA 94, 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haston, W. S., Shields, J. M. & Wilkinson, P. C. (1983) Exp. Cell Res. 146, 117–126. [DOI] [PubMed] [Google Scholar]
- 7.Lo, C.-M., Wang, H.-B., Dembo, M. & Wang, Y.-L. (2000) Biophys. J. 79, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong, J. Y., Velasco, A., Rajagopalan, P. & Pham, Q. (2003) Langmuir 19, 1908–1913. [Google Scholar]
- 9.Bell, E., Ivarsson, B. & Merrill, C. (1979) Proc. Natl. Acad. Sci. USA 76, 1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eastwood, M., Mudera, V. C., McGrouther, D. A. & Brown, R. A. (1998) Cell Motil. Cytoskeleton 40, 13–21. [DOI] [PubMed] [Google Scholar]
- 11.Takakuda, K. & Miyairi, H. (1996) Biomaterials 17, 1393–1397. [DOI] [PubMed] [Google Scholar]
- 12.Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. (2001) Nat. Rev. Mol. Cell. Biol. 2, 793–805. [DOI] [PubMed] [Google Scholar]
- 13.Wang, N., Butler, J. P. & Ingber, D. E. (1993) Science 260, 1124–1127. [DOI] [PubMed] [Google Scholar]
- 14.Choquet, D., Felsenfeld, D. F. & Sheetz, M. P. (1997) Cell 88, 39–48. [DOI] [PubMed] [Google Scholar]
- 15.Riveline, D., Zamir, E., Balaban, N. Q., Schwarz, U. S., Geiger, B., Kam, Z. & Bershadsky, A. D. (2001) J. Cell Biol. 153, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balaban, N. Q., Schwarz, U. S., Riveline, D., Goichberg, P., Tzur, G., Sabanay, I., Mahalu, D., Safran, S., Bershadsky, A., Addadi, L. & Geiger, B. (2001) Nat. Cell Biol. 3, 466–472. [DOI] [PubMed] [Google Scholar]
- 17.Tan, J. L., Tien, J., Pirone, D. M., Gray, D. S., Bhadriraju, K. & Chen, C. S. (2003) Proc. Natl. Acad. Sci. USA 100, 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cukierman, E., Pankov, R., Stevens, D. R. & Yamada, K. M. (2002) Science 294, 1708–1712. [DOI] [PubMed] [Google Scholar]
- 19.Siems, R. (1968) Phys. Stat. Sol. 30, 645–658. [Google Scholar]
- 20.Wagner, H. & Horner, H. (1974) Adv. Phys. 23, 587. [Google Scholar]
- 21.Schwarz, U. S. & Safran, S. A. (2002) Phys. Rev. Lett. 88, 048102. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz, U. S., Balaban, N. Q., Riveline, D., Bershadsky, A., Geiger, B. & Safran, S. A. (2002) Biophys. J. 83, 1380–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walpole, L. J. (1996) Int. J. Eng. Sci. 34, 629–638. [Google Scholar]
- 24.Grinnell, F. (2000) Trends Cell Biol. 10, 362–365. [DOI] [PubMed] [Google Scholar]
- 25.Hirsekorn, R.-P. & Siems, R. (1981) Z. Phys. B Condens. Matter 40, 311–319. [Google Scholar]
- 26.Moon, A. G. & Tranquillo, R. T. (1993) AIChE J. 39, 163–177. [Google Scholar]
- 27.Oster, G. F., Murray, J. D. & Harris, A. K. (1983) J. Embryol. Exp. Morphol. 78, 83–125. [PubMed] [Google Scholar]
- 28.Barocas, V. H. & Tranquillo, R. T. (1997) J. Biomech. Eng. 119, 137–145. [DOI] [PubMed] [Google Scholar]
- 29.Girton, T. S., Barocas, V. H. & Tranquillo, R. T. (2002) J. Biomech. Eng. 124, 568–575. [DOI] [PubMed] [Google Scholar]
- 30.Mandeville, J. T. H., Lawson, M. A. & Maxfield, F. R. (1997) J. Leukocyte Biol. 61, 188–200. [DOI] [PubMed] [Google Scholar]
- 31.Tlusty, T. & Safran, S. A. (2000) Science 290, 1328–1331. [DOI] [PubMed] [Google Scholar]