Abstract
Adult bone marrow and skeletal muscle have been shown to contain a subpopulation of cells, called side population (SP) cells, that can be isolated with the fluorescence-activated cell sorter. We used a similar method to identify SP cells in the skin of adult mice. These cells express surface markers similar to SP cells isolated from skeletal muscle, but differ from bone marrow SP cells and do not express hematopoietic markers. When transplanted into nonirradiated mdx mice, nuclei from donor skin SP cells are found within myofibers that express dystrophin. Thus, adult skin SP cells can engraft in dystrophic skeletal muscle even in the absence of total body irradiation.
Tissues that undergo rapid renewal, such as the skin, the hematopoietic system, and the intestinal epithelia, have been known to harbor stem cells throughout an animal's life span (1–3). Adult stem cells with the potential to give rise to progenitor cells of multiple lineages have been described in the hematopoietic system (4–8) as well as in tissues where the demand for renewal is not as heavy, such as skeletal muscle (5–8) and the central nervous system (9, 10). The ability of these adult stem cells to differentiate and be integrated into multiple tissues offers new insights into cellular plasticity, commitment, and differentiation, and opens the door for cell-based therapy.
The skin is a particularly attractive source of stem cells to address both developmental and therapeutic questions. Its structure and cells are well characterized, and stem cells have been found to be present in both the epidermis and dermis (11–14). Methods for the isolation of specific cell types have been developed and media formulations for in vitro expansion of both mouse and human skin cells are available. Finally, skin is easily accessible for transplant and gene therapy in humans. However, purification of skin stem cells has proven difficult. It often requires an in vitro culture period (13, 15, 16) with unknown effects on stem cell function, or relies on differences in the levels of expression of surface markers also found on more differentiated cells (17).
A technique based on the exclusion of the DNA binding dye Hoechst 33342 has been used to identify and purify, using a fluorescence-activated cell sorter (FACS), a subpopulation of cells, termed side population or SP cells, from both adult bone marrow (5, 18) and skeletal muscle (5). When introduced into the circulation of lethally irradiated mdx mice, an animal model for Duchenne muscular dystrophy (19), these cells have been shown to be able to regenerate the entire hematopoietic compartment and engraft in muscle by fusing with dystrophic muscle fibers (5). We therefore asked whether the same methodology could be used to purify SP cells from the adult mouse skin and whether they might be able to engraft in mdx muscle in vivo without additional irradiation damage.
Materials and Methods
Isolation of SP Cells. Male C57BL/6 and C57BL/10SnJ mice (6–24 weeks old; The Jackson Laboratory) were used. Bone marrow and muscle SP cells were isolated as described (5, 18). For the isolation of skin SP cells, mice were shaved and the dorsal and ventral skin was removed. Subcutaneous (s.c.) fat and muscle tissues were removed by scraping. The skin was minced and digested for 45 min at 37°C in 2 mg/ml collagenase IV and 1.2 units/ml dispase (Worthington). Trypsin was not used because most cell surface markers studied here were sensitive to trypsin digestion, possibly explaining previously reported data (20). Cells were resuspended at 106 cells per ml in PBS with 0.5% BSA (Sigma) and incubated in 12.5 μg/ml Hoechst dye 33342 (Sigma) for 1 h at 37°C. An aliquot was stained with Hoechst in the presence of 100 μM verapamil. Propidium iodide (2 μg/ml) was added before FACS analysis. For injection into mdx mice, gates were placed around SP cells and the bulk of MP cells. Sorted cells were collected in sterile PBS. For antibody staining, cells were incubated on ice for 10 min in the presence of primary antibody (4 μg per 106 cells), washed, and then incubated for 10 min on ice with phycoerythrin-conjugated streptavidin or FITC-conjugated mouse anti-rat secondary antibody. Monoclonal antibodies used: anti-integrin α6 (GoH3 clone), biotin-anti-Sca-1 (E13–161.7 clone), biotin-anti-CD34 (RAM34 clone), biotin-anti-CD43 (S7 clone), biotin-anti-CD45 (30-F11 clone), biotin-anti-c-Kit (2B8 clone), biotin-anti-CD71 (C2 clone) (PharMingen). FACS analysis and cell sorting were performed on a FACSVantage SE flow sorter (Becton Dickinson) as described (5).
Isolation of Dermis and Epidermis. Skin epidermal samples were prepared as described (21). After removal of the epidermis, the dermis was minced and incubated in 2 mg/ml collagenase IV and 1.2 units/ml dispase (Worthington) for 30 min at 37°C.
Histology. Skin and peeled dermis samples were fixed in 4% paraformaldehyde (EM Sciences), infused with 0.5 M sucrose (Sigma), and frozen in liquid nitrogen. Ten-micrometer cryosections were postfixed in 4% paraformaldehyde and processed for hematoxylin-eosin staining.
Fluorescence in Situ Hybridization (FISH) Analysis. Skin SP cells from 6- to 10-week-old male C57BL/10SnJ mice were injected into the tail vein of 6-week-old C57BL/10ScSn-Dmd-mdx female mice (The Jackson Laboratory). Three months later, muscles were harvested and fresh-frozen in liquid nitrogen. Preparation of the Y-chromosome probe (a kind gift of E. Snyder, Harvard Medical School), immunohistochemistry for dystrophin with the 6–10 antibody, and in situ hybridization were performed as described (5). Control male muscle sections were also hybridized to determine probe efficiency, defined as the percentage of Y-chromosome-positive nuclei for 200–400 total male nuclei. Probes that were 230–310 bp long, had an efficiency of hybridization >48%, and showed a high signal with minimal background were used. Specificity of the signal observed on a 4′,6-diamidino-2-phenylindole (DAPI)-labeled nucleus was first assayed by ensuring that signal could be detected only in the rhodamine filter and not in the fluorescein filter. Signals that were scored as specific were confirmed by using the more sensitive charge-coupled device (CCD) camera. To test whether the signal coincided with the DAPI-labeled DNA, pictures were taken in sequential focal planes. Any signal that was not in the same plane of focus as the DAPI-labeled DNA was discarded. When this screening method was used, no FISH signal was detected in control noninjected female mice. Consecutive sections were examined under a Zeiss Axioplan microscope, and images were collected by using a CCD camera. For total fiber counts two additional consecutive sections were stained with a monoclonal antibody to the laminin α2 chain (clone 4H8-2; Sigma). Low-power pictures were assembled by using photo-shop (Adobe Systems, Mountain View, CA) to reconstruct the entire muscle and count individual fibers.
Western Blot Analysis. Sorted cells were lysed in reducing sample buffer (50 mM Tris·HCl, pH 6.8/2% SDS/0.1% bromophenol blue/10% glycerol/100 mM DTT), and proteins were separated on a precast Novex 4–12% Tris-Glycine gel (Invitrogen) then transferred on a nitrocellulose membrane. Cytokeratins were detected by using an anti-pan-cytokeratin antibody (clone C-11; Abcam, Cambridge, U.K.) at 1:500 dilution followed by HRP-conjugated donkey-anti-mouse secondary antibody (Jackson ImmunoResearch) and enhanced chemiluminescence (Perkin–Elmer).
Results
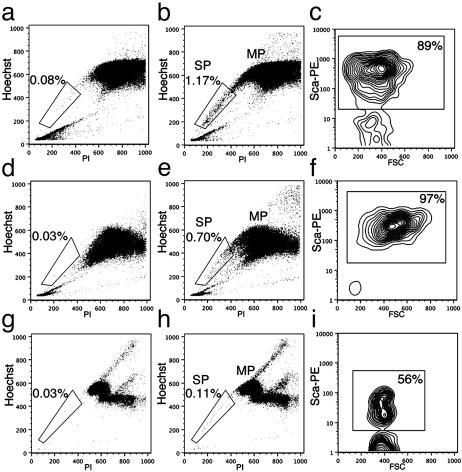
Isolation of SP Cells from Adult Skin. To determine whether SP cells with low Hoechst dye 33342 incorporation are present in the skin, the procedure developed for skeletal muscle (5) was adapted to dissociate and label dermal and epidermal cells from adult mouse skin. To avoid contamination of skin preparations by s.c. skeletal muscles, the skin was stretched and s.c. fat and muscle were carefully and completely removed by scraping. In an effort to ensure the preservation of surface proteins and the functionality of the transporter responsible for Hoechst efflux, the same relatively mild enzyme mix previously used for muscle (5) was also used to dissociate skin. Dissociated cells were labeled with Hoechst dye 33342 in the presence or absence of verapamil, an inhibitor of multidrug resistance (MDR) and MDR-like transporters (Fig. 1 a and b). Verapamil has been shown to prevent Hoechst dye 33342 exclusion from muscle and bone marrow SP cells resulting in their disappearance from the FACS profile (5, 18) (Fig. 1 d and g). Samples were also briefly labeled with propidium iodide to visualize and exclude dead cells during FACS analysis. Bone marrow and skeletal muscle cells were also isolated and labeled with Hoechst dye 33342 for comparison (Fig. 1 d, e, g, and h). FACS analysis of live cells revealed the presence of two major populations of cells in all samples: a large population with high Hoechst fluorescence referred to as main population (MP) and a rarer population of cells with low Hoechst fluorescence corresponding to the side population (SP; Fig. 1 b, e, and h). When labeled in the presence of verapamil, adult skin SP cells are also unable to exclude Hoechst dye 33342 and are no longer visible in the FACS profile (Fig. 1a). Skin SP cells range between 0.5% and 2.5% of total live cells, and can be isolated from mice as old as 6 months of age. Therefore, adult mouse skin contains SP cells with similar physical properties as SP cells present in bone marrow and skeletal muscle.
Fig. 1.
Identification of adult mouse skin SP cells and comparison to skeletal muscle and bone marrow SP cells. FACS profiles of live mononuclear cells isolated from adult skin (a–c), skeletal muscle (d–f), and bone marrow (g–i). SP and MP cells are present in all tissues examined (b, e, and h), and addition of verapamil (a, d, and g) selectively prevented Hoechst exclusion from SP cells. A gate was set around SP cells, and expression of the cell surface marker Sca-1 was analyzed for SP cells from each tissue (c, f, and i). Negative controls where the primary antibody was omitted were used to determine background levels of fluorescence and to set the gate around Sca-1-positive SP cells. The percentage of cells within the SP gate (a, b, d, e, g, and h) or the Sca-1-positive gate (c, f, and i) is indicated. PI, propidium iodide.
Comparison of Surface Markers on SP Cells from Skin, Skeletal Muscle, and Bone Marrow. The expression of Sca-1 and CD34, as well as four surface markers usually expressed by cells of the hematopoietic lineage: c-kit, CD43, CD44, and CD45, was compared between skin, skeletal muscle, and bone marrow SP cells. Cells were first stained with Hoechst 33342 followed by antibody staining to cell surface markers. The percentage of SP cells positive for each marker studied was determined by comparison to a sample stained with an isotype control antibody. Examples for SP cells from skin, skeletal muscle, and bone marrow labeled with an antibody to Sca-1 are shown in Fig. 1 c, f, and i. Skin and skeletal muscle SP cells have very similar expression patterns for all markers studied (Table 1) and form a relatively homogeneous population of cells positive for CD34 and Sca-1, but negative for hematopoietic markers. By contrast, a large proportion of bone marrow SP cells expresses hematopoietic markers, whereas only a very small number is CD34-positive (54%) and ≈56% of cells are Sca-1-positive (Table 1). This finding indicates the presence of at least two separate populations of cells within bone marrow SP cells. Increasing the concentration of the Sca-1 antibody up to 20 μg per 106 cells did not change this result, indicating that the antibody was properly titrated (data not shown). Expression of Sca-1, however, was very variable between individual mice, as denoted by the large standard deviation (Table 1) and fluctuated between 25% and 78%, possibly explaining the discrepancy with previously published results (22). Enzymatic digestion did not affect the integrity of the surface antigens studied because cells reactive for CD34, Sca-1, and the hematopoietic markers were found among skin and muscle MP cells (see Table 3, which is published as supporting information on the PNAS web site, www.pnas.org). In addition, enzymatic digestion of bone marrow cells had no effect on the percentage of cells that stained positive for these antibodies (data not shown). It should be noted that the expression pattern of all of the above mentioned markers is not unique to SP cells, and that in all tissues a significant fraction of the MP cells was also similarly positive or negative for the markers studied (Table 3). Muscle and skin SP cells further differed from bone marrow SP in that the optimum concentration of Hoechst dye 33342 for skin and muscle samples was toxic to bone marrow SP cells (Fig. 6, which is published as supporting information on the PNAS web site). Indeed, staining of bone marrow cells with the Hoechst concentration optimal for muscle and skin (12.5 μg/ml) resulted in a 30-fold decrease in bone marrow SP cells (0.9% at 5 μg/ml Hoechst versus 0.03% at 12.5 μg/ml Hoechst). Taken together, these results suggest that skin and muscle SP cells are very similar to each other but distinct from bone marrow SP cells both in terms of surface marker expression and resistance to Hoechst-induced toxicity.
Table 1. Comparison of surface marker expression on skin, skeletal muscle, and bone marrow SP cells as determined by FACS analysis.
| SP cells | Sca-1 | CD34 | c-Kit | CD43 | CD44 | CD45 |
|---|---|---|---|---|---|---|
| Skin | 92 ± 5 (4) | 91 ± 3 (2) | 2 ± 1 (3) | 0.0 ± 0.0 (2) | 0.1 ± 0.2 (3) | 0.6 ± 0.8 (2) |
| Muscle | 94 ± 3 (4) | 81 ± 3 (5) | 2 ± 3 (3) | 0.2 ± 0.5 (4) | 0.0 ± 0.0 (2) | 1 ± 2 (4) |
| Bone marrow | 56 ± 19 (15) | 54 ± 9 (6) | 68 ± 4 (5) | 88 ± 7 (2) | 94 ± 6 (3) | 98 ± 3 (5) |
Numbers indicate the mean percentage ± standard deviation of positive cells within the SP gate. The number of independent experiments is indicated in parentheses.
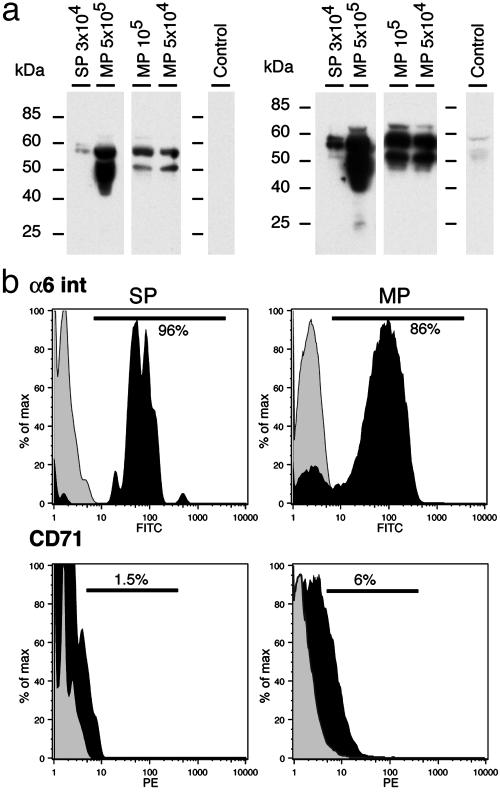
Epidermal Versus Dermal Localization of Skin SP Cells. To determine whether skin SP cells are dermal or epidermal in origin, the expression of cytokeratins as well as cell surface markers found on epidermal (keratinocyte) stem cells was studied, then dermal and epidermal samples were obtained and analyzed for the presence of SP cells. Cytokeratin expression was determined on purified skin SP and MP cells by Western blot using a pan-cytokeratin antibody. Because only a small number of SP cells could be purified for each experiment, MP cells were divided into aliquots of 5 × 104, 105, and 5 × 105 cells before protein extraction to determine whether the antibody could adequately detect cytokeratins in protein extracts derived from small samples. Two main broad bands at 58 kDa and 52 kDa could be seen in all MP samples, even when as few as 50,000 cells were used (Fig. 2a). Purified SP cells also showed cytokeratin expression but with a different pattern than MP cells (Fig. 2a). Two bands of ≈58 kDa were present in SP cells, whereas the 52-kDa band was absent. At longer exposures (Fig. 2a Right) a faint band at 52 kDa was seen in the SP sample and could represent low-level expression of this cytokeratin. However, a similar faint band is also present in the control sample where the primary antibody was omitted, suggesting that the 52-kDa band present in the SP sample is likely nonspecific.
Fig. 2.
Skin SP cells express cytokeratins and integrin α6 but not CD71. (a) Western blot analysis of cytokeratin expression in skin SP and MP cells. Proteins were extracted from different numbers of MP cells to take into account the smaller number of SP cells that could be purified from any individual mouse. Short (Left) and long (Right) exposure times for the same Western blot show that skin SP cells express only a subset of cytokeratins found in skin MP cells. In the control lane, protein extract from 5 × 105 MP cells was run and incubated in the presence of the secondary antibody alone. (b) FACS analysis of skin cells double labeled with Hoechst dye 33342 and monoclonal antibodies to the integrin α6 subunit (Upper) or CD71 (Lower). Expression levels of these markers were determined for gated SP (Left) and MP (Right) cells and are shown in black. Control samples where the primary antibody was omitted or replaced by an irrelevant IgG are shown in light gray. Bars indicate the position of the gates used for calculating the percentage of cells positive for the marker studied.
It has been previously reported that keratinocyte stem cells are integrin α6-bright and CD71-negative (17). The expression of the integrin α6 subunit and of the transferrin receptor (CD71) was assessed by FACS in our whole skin preparations. Almost all skin SP (96%) cells were found to express high levels of integrin α6 subunit and only a very small percentage (1.5%) expressed very low levels of CD71 (Fig. 2b). These features however were not unique to skin SP cells, because in preparations of whole skin, a majority of MP cells also expressed high levels of integrin α6 (86%) and only a minority were weakly positive for CD71 (6%; Fig. 2b).
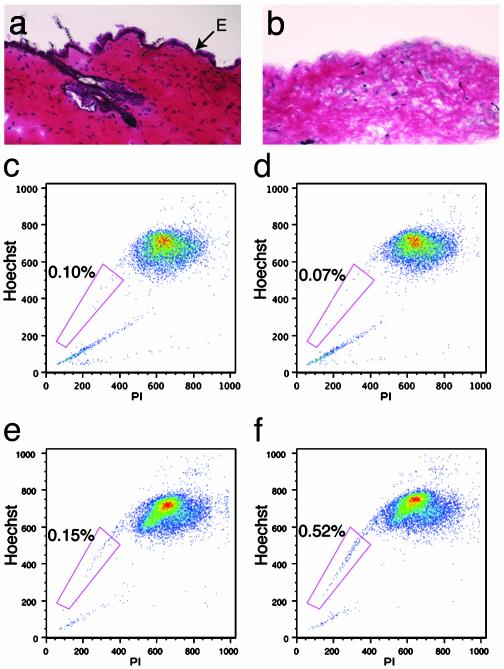
To determine whether skin SP cells reside in the epidermal layer, skin fractions enriched in either epidermal or dermal cells were prepared by using the method described by Morris et al. (21) for adult mouse skin, and processed for FACS analysis. A sample of the dermis was set-aside after peeling to confirm by histological methods the complete removal of the epidermal layer. Keratinocytes and hair follicles were present in intact skin (Fig. 3a) but absent in dermal samples after peeling (Fig. 3b). Close examination of the remaining dermis indicated a paucity of hematoxylin-labeled nuclei, suggesting that a fraction of dermal cells had likely been removed with the epidermal layer (Fig. 3b). FACS analysis of dermal (Fig. 3d) and epidermal (Fig. 3f) enriched fractions revealed the presence of SP cells only in the epidermal sample. These results indicate that skin SP cells are present in skin preparations used to enrich for keratinocytes. Interestingly, the trypsin treatment required for the separation of the two layers did not significantly affect the ability of skin SP cells to efflux Hoechst dye 33342 but slightly decreased the blocking efficiency of verapamil, suggesting that the multidrug resistance-like transporter responsible for Hoechst efflux in these cells is resistant to trypsin.
Fig. 3.
SP cells are present in fractions enriched for epidermal cells. Sections of intact skin (a) and dermis after peeling (b) were stained by hematoxylin/eosin to confirm complete removal of the epidermal layer (E). Keratinocytes associated with hair follicles have also been peeled off. FACS analysis of cells isolated from the dermis (c and d) or the epidermis (e and f) shows that SP cells are present only in the fraction enriched for epidermal cells (f). Inhibition of Hoechst dye 33342 efflux by verapamil (c and e) was decreased by trypsin digestion of the tissue. PI, propidium iodide.
Skin SP Cells Can Engraft in Skeletal Muscle in Vivo. To determine the in vivo potential of skin cells to engraft in skeletal muscle, skin SP and MP cells isolated from a wild type male donor were injected into female mdx mice. Mdx mice have a mutation in the dystrophin gene (19) that leads to a mild progressive muscle degeneration. In these mice, skeletal muscles show a lack of dystrophin expression, with the exception of a few revertant fibers (23).
Skin cells were isolated by using the mild enzyme mix rather than trypsin to preserve the integrity of cell surface receptors that might be needed for infiltration of muscle tissues from the circulation and fusion with muscle fibers. For each experiment a portion of the sample was treated with verapamil to properly identify SP cells. SP (6,000–50,000 cells) and MP (300,000 and 800,000 cells) cells were purified and injected intravenously into 6-week-old female mdx mice. Three months after injection, recipient mice were killed and their muscles were analyzed for expression of dystrophin and for the presence of Y-chromosome-bearing nuclei within dystrophin-positive fibers.
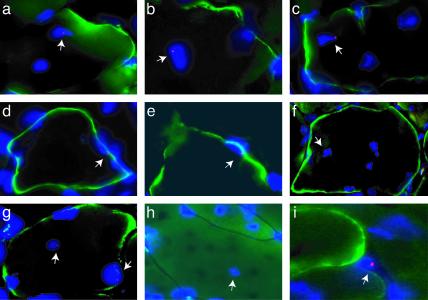
Y-chromosome-positive nuclei satisfying a set of stringent criteria (see Materials and Methods) were found within dystrophin-positive muscle fibers in both the tibialis anterior and triceps brachii muscles (Fig. 4 and Table 2) of mdx female mice injected with skin SP cells. Some such fibers had strong dystrophin expression, whereas others expressed low or undetectable levels of dystrophin expression within the plane of the Y-chromosome-positive nucleus but showed increased dystrophin expression in adjacent sections. Some mononuclear cells were also found to be positive for the Y chromosome. They were usually close to muscle fibers but not in a position suggestive of satellite cells (Fig. 4i). Donor nuclei were not associated with regions of mononuclear cell infiltration, suggesting that these were not circulating cells. Consistent with this observation, no donor cells were found in the spleen of animals injected with skin SP or MP cells (data not shown), an organ in which circulating cells are known to transit. Thus in nonirradiated hosts, skin SP cells did not differentiate into hematopoietic cells and likely did not remain in the circulation. However, they infiltrated damaged muscles and took residence there. Quantification of Y-chromosome-positive nuclei and dystrophin-positive fibers was performed for the triceps brachii muscle of each mouse over an 80-μm muscle segment. Skin SP cells were able to infiltrate damaged muscle and fuse with preexisting muscle fibers (Table 2). More than 9% of dystrophin-positive fibers contained a Y-chromosome-positive nucleus in mice injected with >6,000 skin SP cells. This percentage is comparable to that previously reported for irradiated mdx mice 1 month after i.v. injection of an equivalent number of muscle SP cells (5). Furthermore, skin MP cells showed almost no incorporation into muscle fibers even though 60 times more MP cells were injected, strongly suggesting that our procedure did enrich for cells with muscle engraftment potential and that fusion events with muscle fibers did not occur at random.
Fig. 4.
Skin SP cells participate in muscle repair in the mdx mouse. Male donor-derived skin SP cells have fused with dystrophin-positive mdx muscle fibers. Nuclei bearing a Y chromosome were detected by FISH in triceps (arrows in a–c and g–i) and tibialis anterior (arrows in d–f) muscles in all three female mdx mice injected with 12,000–50,000 skin SP cells. Note that Y-chromosome-positive nuclei are present within dystrophin-expressing muscle fibers in both central and peripheral positions. Some muscle fibers have incorporated more than one donor nucleus (g). FISH signal is also detected in mononuclear cells (i) and myofibers with no or low levels of dystrophin expression (h). Red, Y chromosome; blue, nuclei; green, dystrophin.
Table 2. Quantification of Y-chromosome-positive nuclei within dystrophin-positive muscle fibers in the triceps brachii of female mdx mice.
| Mouse ID | Cells | No. of cells | Sections | Total | Max D+ | D+Y+ |
|---|---|---|---|---|---|---|
| 73570Y | SSP | 50,000 | 8 | 4,420 | 93 | 9 |
| 73570Z | SSP | 13,000 | 8 | 3,066 | 95 | 9 |
| 73550C | SSP | 12,000 | 8 | 3,407 | 52 | 5 |
| 73570U | SSP | 6,000 | 8 | 6,632 | 155 | 1 |
| 73570T | SMP | 800,000 | 8 | 3,255 | 34 | 0 |
| 735795B | SMP | 300,000 | 8 | 3,296 | 186 | 1 |
| Control | None | — | 5 | 4,700 | 136 | 0 |
Each mouse injected with SSP cells represents an independent experiment. Total, total number of fibers per section; Max D+, maximum number of dystrophin-positive fibers per section; D+Y+, dystrophin-positive fibers that contain at least one Y-chromosome-positive nucleus as determined by FISH; SSP, skin SP; SMP, skin MP.
Comparison of the percentage of dystrophin-positive muscle fibers for the tibialis anterior and triceps brachii of all experimental mice showed no therapeutically significant restoration of dystrophin expression in the muscles of mice treated with skin SP cells. However, the number of revertant fibers shows great variability between animals and as well as within the same animal. This variability masked any marginal effect that donor cells might have had on dystrophin expression and renders impossible an accurate estimate of the impact of SP cell transplantation in these animals. Analysis of the distribution of the dystrophin-positive fibers containing a donor nucleus within the 80 μm segment (Fig. 6) reveals the incorporation of donor cells mainly into isolated dystrophin-positive fibers and occasionally in clustered fibers likely to be dystrophin revertants. Unlike revertant fibers, isolated fibers containing a donor nucleus usually show dystrophin expression only over a portion of the 80-μm segment studied. In some cases, dystrophin expression in these fibers was localized to the portion of sarcolemma close to the donor nucleus (Fig. 5b Lower), suggesting that dystrophin expression is likely driven by the donor nucleus and not the result of spontaneous reversion. The overall distribution of donor cells in these muscles also shows that the entire muscle was infiltrated at multiple sites (Fig. 5).
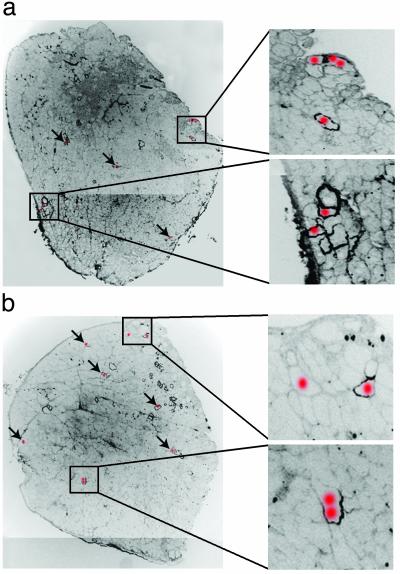
Fig. 5.
Distribution of Y-chromosome-positive nuclei in the triceps muscles of female mdx mice injected with male skin SP cells. One representative section of the eight consecutive sections analyzed by FISH and dystrophin immunohistochemistry was assembled to show the overall distribution of dystrophin-positive fibers. A red dot over a dystrophin-positive myofiber indicates that in one of the eight sections a Y-chromosome-positive nucleus was found at that location. Arrows indicate where donor nuclei are located in the low-power pictures. Dystrophin staining is shown in black. Pictures were overexposed to allow visualization of muscle histology. Donor nuclei were present in clustered or isolated dystrophin-positive fibers. Dystrophin expression in these fibers ranged from very high to low, encompassing the entire fiber diameter or only the region of sarcolemma closest to the site of incorporation of the donor nucleus (Insets). Some fibers showed no or extremely low dystrophin expression in the plane of the donor nucleus but were dystrophin-positive in the adjacent section, suggesting the formation of nuclear domains for dystrophin expression that either were centered around the donor nucleus or were skewed in one direction. Mice in a and b received 50,000 and 13,000 skin SP cells, respectively.
Discussion
The data presented here describe the isolation of SP cells from adult mouse skin and provide a comparison of some cell surface markers with previously described SP cells isolated from adult bone marrow and skeletal muscle. Skin SP cells were enriched in epidermal preparations and found to express cytokeratins, suggesting that they might represent a subpopulation of keratinocytes. Finally, i.v. injection of male skin SP cells into female mdx mice resulted in their incorporation into dystrophic muscle fibers. Following adaptation of protocols for the isolation of SP cells from bone marrow and muscle tissues (5, 18), the existence of a relatively abundant (0.5–2.5%) population of SP cells in adult mouse skin was demonstrated. The percentage of SP cells was consistently higher than for bone marrow, which varied between 0.03% and 0.1%. Furthermore, the percentage of skin SP cells did not correlate with the age of the animal (6 weeks to 6 months), possibly because of the significant demand on this tissue for renewal and wound repair. Given the accessibility of the skin, its higher content in SP cells, and the flexibility on sampling age, this tissue should prove a good source of cells for transplantation. Comparison of skin SP cells to bone marrow and muscle SP cells for the expression of surface markers clearly shows that skin SP cells are very similar to muscle SP cells and differ significantly from bone marrow SP cells. The hematopoietic markers CD45, CD44, CD43, and c-kit are not expressed on the majority of skin and muscle SP cells indicating that these cells are not part of the hematopoietic system (24) and are not likely to be contaminating peripheral blood SP cells. This is further supported by the observation that skin and muscle SP cells are much more resistant to Hoechst dye 33342 toxicity than bone marrow SP cells. Thus, circulating bone marrow-derived SP cells present in muscle or skin would most likely be killed during sample preparation and would not contribute to the SP population observed in these tissues. Therefore, our data favor the notion that skin and muscle SP cells reside within skin and muscle respectively, but do not address directly the issue of their developmental origin.
Stem cells of epidermal and dermal origin have been previously described (11–14), raising the question of their relationship to skin SP cells. In a series of preliminary experiments, the culture medium used to expand dermal stem cells (13) did not induce the formation of spheres by isolated skin SP cells. The observation that skin SP cells are enriched in epidermal and not dermal fractions further supports the notion that skin SP cells are different from dermal stem cells, and suggests that they are not related to the arrector pili, a smooth muscle located in the dermis. Although the absence of c-Kit expression on their surface suggests that they are not melanocytes, the expression of high levels of the integrin α6 subunit and especially cytokeratins indicates that skin SP cells might be a keratinocyte subpopulation. Recent studies (25) have shown that keratinocyte stem cells are capable of excluding Hoechst and can contribute to all cell lineages when injected into blastocysts. Whether skin SP cells are identical to or a subpopulation of keratinocyte stem cells remains to be determined. Further studies will be needed to explore the full in vivo and in vitro potential of skin SP cells to give rise to multiple lineages and whether they satisfy all of the criteria for stem cells.
After i.v. injection into mdx mice, skin SP cells, but not MP cells, were able to infiltrate dystrophic muscle, and either themselves or their daughter cells were able to fuse with muscle fibers. The near complete lack of muscle conversion of skin MP cells (<0.5% of dystrophin-positive myofibers contained a donor nucleus), even when 800,000 cells were injected, sharply contrasts with the observation of fused donor nuclei in the muscles of mice injected with as few as 12,000 skin SP cells (≈9% of dystrophin-positive myofibers contained a donor nucleus). This observation alone demonstrates the enhanced ability of skin SP compared with MP cells for muscle engraftment, and validates our purification method.
Muscle engraftment of skin SP cells could be the end result of different mechanisms. One possibility is that skin SP cells are indeed a keratinocyte subpopulation and they undergo a nuclear reprogramming triggered either by factors in the muscle environment or by the fusion event with a muscle fiber. Previous studies (26) have shown that nuclear reprogramming can occur in keratinocytes on fusion with muscle fibers in vitro resulting in the expression of muscle genes. Another possibility is that rare events of cellular fusion occur between a skin SP cell and a myoblast leading to nuclear fusion and incorporation of the donor cell into muscle fibers. This phenomenon of nuclear fusion has been reported to occur in vitro between embryonic stem cells and either bone marrow cells (27) or neural progenitors (28). Nuclear fusion, however, might be more prone to occur in vitro than in vivo because cellular fusion without nuclear fusion has been reported in vivo in skeletal muscle after bone marrow transplantation (29). Whether nuclear fusion occurs or not between skin SP cells and myonuclei as well as whether this has any bearing on the restoration of full-length dystrophin expression in dystrophic muscle, are two questions that will need to be further investigated.
Recent studies (30) have shown that homing of bone marrow cells to skeletal muscle in wild-type mice is enhanced by damage from total body irradiation alone or in conjunction with exercise. In our study, mdx mice were neither irradiated nor exercised, yet donor nuclei were found within muscle fibers. It is therefore likely that skin SP cells can respond to signals released by chronic muscle damage. This provides hope that recruitment and incorporation of skin SP cells into muscle could be increased by combining cell transplantation with exercise regiments, or by identifying factors that would prime skin SP cells toward a muscle lineage and/or facilitate their incorporation into muscle.
In summary, our results demonstrate that SP cells with characteristics very similar to muscle SP cells can be purified from the skin, a much more accessible tissue. Upon i.v. injection, these cells can reach dystrophic muscle even in the absence of total body irradiation. Once inside the muscle, skin SP cells can fuse with myofibers and possibly restore full-length dystrophin expression. Future studies should be aimed at exploring more in depth the biological characteristics of skin SP cells and improving their incorporation into dystrophic muscle reaching therapeutically relevant levels.
Supplementary Material
Acknowledgments
We thank Drs. Emanuela Gussoni, Carlos Miranda, and Jeffrey Guyon for critical review of the manuscript. We are also grateful to Dr. Evan Snyder for his generous gift of the Y-chromosome probe. This work was supported by a grant from the Muscular Dystrophy Association and a generous contribution of the Bernard F. and Alva B. Gimbel Foundation. L.M.K. is an investigator with the Howard Hughes Medical Institute.
Abbreviations: SP, side population; MP, main population; FACS, fluorescence-activated cell sorter; FISH, fluorescence in situ hybridization.
References
- 1.Lord, B. (1997) in Stem Cells, ed. Potten, C. S. (Academic, London), pp. 401–422.
- 2.Miller, S. J., Sun, T.-T. & Lavker, R. M. (1997) in Stem Cells, ed. Potten, C. S. (Academic, London), pp. 331–400.
- 3.Wright, N. A. (1997) in Stem Cells, ed. Potten, C. S. (Academic, London), pp. 315–330.
- 4.Ferrari, G., Cusella-De Angelis, G., Coletta, M., Paolucci, E., Stornaiuolo, A., Cossu, G. & Mavilio, F. (1998) Science 279, 1528–1530. [DOI] [PubMed] [Google Scholar]
- 5.Gussoni, E., Soneoka, Y., Strickland, C. D., Buzney, E. A., Khan, M. K., Flint, A. F., Kunkel, L. M. & Mulligan, R. C. (1999) Nature 401, 390–394. [DOI] [PubMed] [Google Scholar]
- 6.Brazelton, T. R., Rossi, F. M., Keshet, G. I. & Blau, H. M. (2000) Science 290, 1775–1779. [DOI] [PubMed] [Google Scholar]
- 7.Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. (2000) Science 290, 1779–1782. [DOI] [PubMed] [Google Scholar]
- 8.Orlic, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S. M., Li, B., Pickel, J., McKay, R., Nadal-Ginard, B., Bodine, D. M., et al. (2001) Nature 410, 701–705. [DOI] [PubMed] [Google Scholar]
- 9.Bjornson, C. R., Rietze, R. L., Reynolds, B. A., Magli, M. C. & Vescovi, A. L. (1999) Science 283, 534–537. [DOI] [PubMed] [Google Scholar]
- 10.Galli, R., Borello, U., Gritti, A., Minasi, M. G., Bjornson, C., Coletta, M., Mora, M., De Angelis, M. G., Fiocco, R., Cossu, G. & Vescovi, A. L. (2000) Nat. Neurosci. 3, 986–991. [DOI] [PubMed] [Google Scholar]
- 11.Cotsarelis, G., Sun, T.-T. & Lavker, R. M. (1990) Cell 61, 1329–1337. [DOI] [PubMed] [Google Scholar]
- 12.Ghazizadeh, S. & Taichman, L. B. (2001) EMBO J. 20, 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toma, J. G., Akhavan, M., Fernandes, K. J., Barnabe-Heider, F., Sadikot, A., Kaplan, D. R. & Miller, F. D. (2001) Nat. Cell Biol. 3, 778–784. [DOI] [PubMed] [Google Scholar]
- 14.Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T.-T. & Lavker, R. M. (2000) Cell 102, 451–461. [DOI] [PubMed] [Google Scholar]
- 15.Bickenbach, J. R. & Chism, E. (1998) Exp. Cell Res. 244, 184–195. [DOI] [PubMed] [Google Scholar]
- 16.Jones, P. H. & Watt, F. M. (1993) Cell 73, 713–724. [DOI] [PubMed] [Google Scholar]
- 17.Tani, H., Morris, R. J. & Kaur, P. (2000) Proc. Natl. Acad. Sci. USA 97, 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodell, M. A., Brose, K., Paradis, G., Conner, A. S. & Mulligan, R. C. (1996) J. Exp. Med. 183, 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sicinski, P., Geng, Y., Ryder-Cook, A. S., Barnard, E. A., Darlison, M. G. & Barnard, P. J. (1989) Science 244, 1578–1580. [DOI] [PubMed] [Google Scholar]
- 20.Albert, M. R., Foster, R. A. & Vogel, J. C. (2001) J. Invest. Dermatol. 117, 943–948. [DOI] [PubMed] [Google Scholar]
- 21.Morris, R. J., Fischer, S. M. & Slaga, T. J. (1986) Cancer Res. 46, 3061–3066. [PubMed] [Google Scholar]
- 22.Goodell, M. A., Rosenzweig, M., Kim, H., Marks, D. F., DeMaria, M., Paradis, G., Grupp, S. A., Sieff, C. A., Mulligan, R. C. & Johnson, R. P. (1997) Nat. Med. 3, 1337–1345. [DOI] [PubMed] [Google Scholar]
- 23.Lu, Q. L., Morris, G. E., Wilton, S. D., Ly, T., Artem'yeva, O. V., Strong, P. & Partridge, T. A. (2000) J. Cell Biol. 148, 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trowbridge, I. S. & Thomas, M. L. (1994) Annu. Rev. Immunol. 12, 85–116. [DOI] [PubMed] [Google Scholar]
- 25.Liang, L. & Bickenbach, J. R. (2002) Stem Cells 20, 21–31. [DOI] [PubMed] [Google Scholar]
- 26.Blau, H. M., Pavlath, G. K., Hardeman, E. C., Chiu, C. P., Silberstein, L., Webster, S. G., Miller, S. C. & Webster, C. (1985) Science 230, 758–766. [DOI] [PubMed] [Google Scholar]
- 27.Terada, N., Hamazaki, T., Oka, M., Hoki, M., Mastalerz, D. M., Nakano, Y., Meyer, E. M., Morel, L., Petersen, B. E. & Scott, E. W. (2002) Nature 416, 542–545. [DOI] [PubMed] [Google Scholar]
- 28.Ying, Q. L., Nichols, J., Evans, E. P. & Smith, A. G. (2002) Nature 416, 545–548. [DOI] [PubMed] [Google Scholar]
- 29.Gussoni, E., Bennett, R. R., Muskiewicz, K. R., Meyerrose, T., Nolta, J. A., Gilgoff, I., Stein, J., Chan, Y. M., Lidov, H. G., Bonnemann, C. G., et al. (2002) J. Clin. Invest. 110, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaBarge, M. A. & Blau, H. M. (2002) Cell 111, 589–601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.