Abstract
In addition to its role in causing Müllerian duct regression, Müllerian inhibiting substance (MIS) is implicated in the regulation of steroidogenesis, breast and prostate growth, and ovarian follicle recruitment, all of which are processes controlled or influenced by the hypothalamic–pituitary–gonadal axis. Whereas the direct effect of MIS on gonadal, prostate, and breast cells is under investigation, the ability of MIS to modulate pituitary function, thereby affecting those tissues indirectly, has not yet been studied. Using LβT2 cells, a murine gonadotrope-derived cell line, we have evaluated the effects of MIS on the expression of the gonadotropin genes. We show that both LβT2 cells and adult rat pituitaries express MIS type II receptor (MISRII) mRNA. Within 2 h, follicle-stimulating hormone β subunit (FSHβ) mRNA levels are significantly induced by addition of MIS to LβT2 cells and remain elevated through 8 h of treatment. Transcriptional activation of both the FSHβ and luteinizing hormone β subunit (LHβ) gene promoters was observed by MIS, which enhances the effect of gonadotropin-releasing hormone (GnRH) agonist on the FSHβ gene promoter and synergizes with the GnRH agonist to stimulate LHβ gene promoter activity. Addition of MIS to LβT2 cells stimulates the activity of the rat LHβ gene promoter with as little as 1 μg/ml and in a dose-dependent manner. These studies report both MISRII expression in rat pituitary cells and a gonadotrope-derived cell line and MIS-mediated activation of LHβ and FSHβ gene expression, and suggest that MIS may modulate the hypothalamic–pituitary–gonadal axis at more than one level.
Keywords: luteinizing hormone, follicle-stimulating hormone, pituitary
Müllerian inhibiting substance [MIS, also known as anti-Müllerian hormone or AMH (1)] is a glycoprotein hormone member of the transforming growth factor β (TGFβ) family of growth and differentiation factors and is well known for its role in the regression of Müllerian ducts. Normally, if an embryo is 46XY, the bipotential gonads commit to testes development and MIS begins to be expressed by differentiating Sertoli cells, leading to regression of the Müllerian ducts at 7–10 weeks in humans and 13–17 days in rodents during male embryonal development. In the absence of MIS, the Müllerian ducts differentiate into the female internal reproductive organs: the uterus, fallopian tubes, and upper vagina (reviewed in ref. 2).
MIS continues to be expressed in males well after Müllerian duct regression and persists at high levels even after birth until puberty, with levels inversely correlated with the increase in serum testosterone. Females begin expressing low levels of MIS in ovarian granulosa cells after birth and reach the same serum levels as those of postpubescent boys. Recent studies suggest that there may be several aspects of physiological significance to the continued MIS expression, including effects on gonadal steroidogenesis (3), follicle recruitment (4, 5), and inhibition of prostate and breast cell proliferation (6, 7).
Male mice overexpressing MIS have reduced levels of testosterone and Leydig cell hypoplasia, and are often undervirilized (8). Conversely, mice with null mutations in either MIS or the MIS type II receptor (MISRII) have Leydig cell hyperplasia (9, 10). Indeed, Leydig cells express MISRII, and MIS inhibits Leydig cell testosterone production directly, at least in part by inhibiting the expression of one or more of the steroidogenic enzymes (11–14). MIS and MISRII are expressed in the granulosa cells of developing follicles (15–18), and a role for MIS in gonadal function in the female has also been demonstrated. In addition to the lack of an internal reproductive tract, the ovaries of female transgenic mice that overexpress MIS become largely depleted of germ cells by 2 weeks postnatal and are not detectable in many of the adult mice (8, 11). Subsequent studies have shown that primordial follicles of mice deficient for MIS are recruited to develop at a faster rate than in wild-type mice (5) and that addition of MIS to cultured ovaries inhibits primordial follicle growth (4). These observations suggest that MIS plays a significant role in ovarian function, although the exact mechanisms have yet to be deduced.
A functional hypothalamic–pituitary–gonadal axis is also critical to mammalian reproductive development and function. The biosynthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the pituitary is tightly controlled in the hypothalamic–pituitary–gonadal axis by an interconnected system of stimulatory and inhibitory mechanisms. In addition to major roles for gonadotropin-releasing hormone (GnRH) and gonadal steroids, several members of the TGFβ family modulate gonadotropin production. Among these, the effects of activins and inhibins have been best characterized. Activins and inhibins were first identified based on their ability to stimulate and inhibit, respectively, the release of pituitary FSH (19, 20). Subsequent studies have revealed additional important physiologic roles for these factors in gonadal function, development, and many other systems (reviewed in refs. 21 and 22). The immortalized mouse pituitary gonadotrope-derived cell lines, αT3-1 and LβT2, have been demonstrated to express activin receptors and to be activin responsive (23, 24). More recently, bone morphogenetic proteins (BMPs) 6, 7, and 15 were found to stimulate FSHβ gene transcription and FSH secretion in LβT2 cells and in primary pituitary cultures (25, 26).
In addition to its established effects on gonadal development and function, there have been reports indicating that MIS might have important regulatory interactions at the level of the pituitary (27, 28), which could also be supported by studies of mice overexpressing MIS or deficient in MIS or MISRII (Table 1). Serum FSH levels have been reported to be significantly lower in 4-month-old adult female MIS knockout mice compared with wild-type controls (5). Other studies showed that 2-month-old transgenic MIS-overexpressing male mice had levels of serum LH and FSH elevated by factors of 8- and 1.5-fold, respectively, over those of the corresponding wild-type male mice (12). Although the elevated levels are consistent with the loss of feedback inhibition by reduced levels of estrogens and testosterone observed in the MIS-overexpressing mice, they could also reflect a direct stimulatory effect of MIS on pituitary gonadotropes. Changes in gonadal inhibin production may also occur in these in vivo models and contribute to altered levels of gonadotropins.
Table 1. Hormonal levels in T-MIS and MISKO mice.
| Wild type | T-MIS | MISKO | Reference | |
|---|---|---|---|---|
| T, pg/ml | ||||
| Adult male | 5,331.7 ± 2,057.5 | 435.0 ± 268.2* | 11 | |
| 2-month-old male | 2,150 ± 490 | 1,200 ± 420* | 1,850 ± 490 | 12 |
| Adult female | <16 | <5 | 11 | |
| E2, pg/ml | ||||
| Adult male | 97.2 ± 46.9 | 225.0 ± 76.1 | 11 | |
| 2-month-old male | 20 ± 15 | 7 ± 9* | 12 | |
| Adult female | 152.8 ± 39.8 | 241.2 ± 64.9 | 11 | |
| LH, ng/ml | ||||
| 2-month-old male | 0.44 ± 0.28 | 3.17 ± 0.33* | 0.83 ± 0.61 | 12 |
| FSH, ng/ml | ||||
| 2-month-old male | 35.42 ± 10.42 | 53.13 ± 11.03† | 12 | |
| 25-day-old female | 24.1 ± 5.3 | 15.0 ± 1.8 | 5 | |
| 4-month-old female | 39.7 ± 2.6 | 27.9 ± 2.9* | 5 | |
| 13-month-old female | 28.8 ± 2.6 | 37.9 ± 3.8 | 5 |
T-MIS, transgenic human MIS; MISKO, MIS knockout.
Significantly different from WT (P ≤ 0.05).
Significantly different from WT (P ≤ 0.001).
Given the known effects of activins and inhibins at multiple levels of the hypothalamic–pituitary–gonadal axis, as well as on development, we hypothesized that MIS might have a similarly important regulatory role. Furthermore, the demonstrated actions on gonadotrope function by BMPs, which share signal transduction pathways with MIS (29–31), suggested that gonadotropes might also be capable of responding to MIS. To test this hypothesis, we studied the effects of MIS on FSH and LH production by using the well characterized gonadotrope-derived cell line, LβT2 (32, 33).
Materials and Methods
Chemicals and Reagents. Radionucleotides were purchased from Perkin–Elmer. All other chemicals were obtained from Sigma or Fisher Scientific unless otherwise noted. Female FCS was purchased from Aires Scientific/Biologos (Richardson, TX). Recombinant human MIS was expressed in Chinese hamster ovary cells and secreted into chemically defined serum-free media, as described (34). GnRH agonist (des-Gly10-[d-Ala6]GnRH ethylamide) was purchased from Sigma.
Cell Culture and Animal Studies. LβT2 and αT3-1 cells, generous gifts of Pamela Mellon (University of California at San Diego, La Jolla), were cultured in high-glucose DMEM (Invitrogen Life Technologies) containing 10% female FCS and 1% penicillin-streptomycin mix. Female FCS was used to avoid inadvertent exposure of the cells to MIS, which is present in serum derived from unsexed fetal calves. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2. RNA was harvested from tissues of 3-month-old male and female mice or 200-g male and female rats (Charles River Breeding Laboratories). All studies involving animals were performed in accordance with mandated standards and approved by the Institutional Animal Care and Use Committee.
Western Blot, Northern Blot, and Quantitative Real-Time (QRT)-PCR Analyses. Western analysis was performed with a polyclonal antibody prepared against the kinase domain of the rat MISRII as described (35). For Northern blot analysis, total RNA was isolated by using TRIzol (Invitrogen). RNA (10 μg) samples were denatured with dimethyl sulfoxide and glyoxal at 65°C, separated in a 1.5% agarose gel, blotted overnight onto nylon membranes, and UV cross-linked. Blots were prehybridized with 100 μg/ml sonicated salmon sperm DNA in 50% formamide hybridization solution and hybridized overnight at 65°C with 2 × 106 cpm/ml of an antisense riboprobe against MISRII (14). Blots were washed at 65°C with 0.1× SSC/0.1% SDS and exposed to radiographic film with intensifying screens at –70°C. Blots were reprobed with a human β-actin riboprobe (36) at 65°C and washed at 65°C with 0.1× SCC/0.1% SDS.
QRT-PCR experiments were performed in a SmartCycler (Cepheid, Sunnyvale, CA) by using Invitrogen-designed Lux primer pairs for murine LHβ (CACTTGCTGCTGCTGAGCCCAAG5G-FAM, TGCAGACTGGGCAGAACTCA) and murine FSHβ (GACCGAGCCAGGCAATCTTACGG5C-FAM, CGGCCCAATACCCAGAAAGT), with reagents from the Platinum Quantitative RT-PCR Thermoscript One-Step System (Invitrogen Life Technologies) and 250 ng total RNA. The reverse transcriptase reaction was done for 30 min at 55°C, and PCR was done with 45 cycles at 95°C for 15 s, 55°C for 30 s, and 72°C for 60 s. The relative concentration of the mRNAs was determined by calculating the difference (x) in threshold cycle (Ct) value, which is then interpreted to represent a 2x-fold difference in template concentration. Rat MISRII was detected by reverse transcription–PCR as above with Lux primers (CACATTCGAGACCTGAGCAGCCAGAATG5G-FAM, CAGGGAGTACCAAGGCAAGG), both reverse transcription and annealing being performed at 62°C.
Luciferase Assays. The reporter constructs used were generated by fusing –797/+5 of the rat LHβ gene, –2000/+698 of the rat FSHβ gene, –846/0 of the human glycoprotein α subunit (αGSU) gene, and –1164/+62 of the mouse GnRH receptor (GnRHR) gene to the firefly luciferase cDNA in a pXP2 vector, as described (37–39). LβT2 cells were transiently transfected by electroporation with 2 μg per well of either reporter construct (αGSU-Luc, LHβ-Luc, FSHβ-Luc, GnRHR-Luc) or 2 μg per well of empty vector (pXP2) and 1 μg per well of an SV40-β-galactosidase vector (kindly provided by Annie Ladoux, Institut National de la Santé et de la Recherche Médicale, Toulouse, France). Cells were then seeded into six-well tissue culture plates, followed by incubation at 37°C for 48 h, with appropriate treatment. For transient transfection of αT3-1, cells were divided into six-well tissue culture plates and cultured overnight in DMEM in the absence of serum or antibiotics to a confluence of 60–80%. αT3-1 cells were then transfected by calcium phosphate coprecipitation (40) with 2 μg per well of either LHβ-Luc or pXP2 for 24 h in media containing 2% FBS, followed by incubation with the appropriate treatment. For luciferase activity measurement, LβT2 and αT3-1 cells were washed twice with ice-cold PBS and lysed with 125 mM Tris·HCl/0.5% Triton X-100. After centrifugation at 14,000 × g and 4°C, luciferase and β-galactosidase activities were measured in the supernatants. Luciferase activity was normalized for β-galactosidase activity to correct for transfection efficiency. To study the effect of MIS on basal and GnRH-stimulated activity of gonadotropin subunit or GnRHR gene promoters, transfected cells were cultured for a total of 48 h and were stimulated with the indicated concentrations of MIS for the last 4 or 24 h and/or 100 nM GnRH agonist for the remaining 4 h.
Statistical Analysis. All luciferase reporter studies except the dose–response study comprised at least three independent experiments, each performed in triplicate. Luciferase results were analyzed by one-way ANOVA on repeated measurements from a representative experiment followed by Tukey–Kramer post hoc test with instat 3.0 (GraphPad, San Diego). The QRT-PCR experiments were also assayed at least three times from a representative experiment and were analyzed by Dunnett's post hoc test with prism 3.0 (GraphPad). Significance was assigned at 95% confidence (P < 0.05).
Results
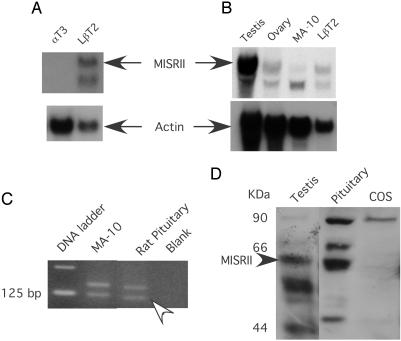
LβT2 Cells and Rat Pituitaries Express the MIS Type II Receptor. To investigate whether MIS might be acting directly at the level of pituitary gonadotropes to regulate the production of LH and FSH, we first looked for expression of MISRII in two mouse gonadotrope-derived cell culture model systems, αT3-1 and LβT2 (Fig. 1A). Northern blot analysis with RNA harvested from αT3-1 and LβT2 cells and using a full-length antisense riboprobe for the rat MISRII indicates that LβT2 cells, but not αT3-1 cells, express the mRNA for the MISRII. RNA from αT3-1 cells did not show expression of the MISRII even with a 6-day exposure of the blot to film. In Fig. 1B, the level of MISRII mRNA in LβT2 cells was compared with that of mouse testes, ovaries, and MA-10 cells, a mouse Leydig cell tumor line that we have used to study the inhibition of steroidogenesis by MIS (41). After overnight exposure, two bands were detected, the smaller of which may be a shortened splice variant with unknown function (42). The expression of full-length MISRII appears to be higher in LβT2 cells than in MA-10 cells, but lower than that observed in either testes or ovaries. MISRII mRNA expression in adult rat pituitaries was not observed by Northern analysis (data not shown) but was detected by reverse transcription–PCR using pituitary RNA isolated from adult female rats as a template (Fig. 1C). MISRII protein was also detected in rat pituitary tissue and testis, but not in COS-7 cells (negative control), by Western analysis with a rabbit polyclonal antibody prepared against the kinase domain of the receptor (Fig. 1D).
Fig. 1.
MISRII mRNA and protein expression in LβT2 cells and rat pituitary. In A and B, total RNA was subjected to Northern blot analysis, probed with an antisense rat MISRII riboprobe, and reprobed with a β-actin riboprobe to control for loading. (A) RNA from αT3-1 cells and LβT2 cells were analyzed as indicated. (B) RNA was harvested from testes and ovaries of mice and from MA-10 cells and LβT2 cells as indicated. (C) Reverse transcription–PCR analysis of RNA from MA-10 and adult female rat pituitaries with primers to MISRII. The arrowhead indicates the MISRII band at the expected size. The unlabeled upper band corresponds to a misprimed MISRII product. Both bands were confirmed by sequencing. (D) Western analysis of protein from adult male testis and pituitary and COS cells with an antibody to the MISRII kinase domain. The MISRII protein is indicated by an arrowhead.
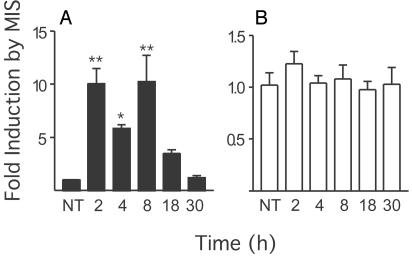
MIS Increases Steady-State mRNA Levels of FSHβ in LβT2 Cells. The expression of the MISRII in the LβT2 gonadotrope cell line suggested that MIS might have effects on gonadotrope function in general, and on gonadotropin production in particular. We next assessed whether addition of MIS to the cells could change the steady-state level of FSHβ and LHβ mRNA by QRT-PCR. We first analyzed FSHβ mRNA and observed that addition of 5 μg/ml (35 nM) MIS significantly increased (P < 0.001) FSHβ mRNA levels ≈10-fold within 2 h (Fig. 2A). The significant induction of FSHβ mRNA by MIS persisted for 8 h. By 18 h, the induction of FSHβ by MIS was no longer significantly different from that of untreated cells, and levels returned to baseline after 30 h. These same RNA samples were also analyzed in parallel for LHβ expression (Fig. 2B). No statistically significant difference in LHβ mRNA levels was observed with MIS addition to the cells. The levels of FSHβ mRNA required six cycles more than LHβ mRNA to reach the threshold value in the QRT-PCR, indicating that ≈64-fold less FSHβ mRNA than LHβ mRNA was present in those samples, assuming equal efficiency of the primers (not shown).
Fig. 2.
MIS regulates endogenous FSHβ mRNA levels. Total RNA was prepared from LβT2 cells treated with 35 nM MIS for the indicated times. Equal amounts of RNA were subjected to combined reverse transcription and QRT-PCR with primers specifictoFSHβ (A) and LHβ (B). The concentration threshold (Ct) values of at least three repeats were averaged, and differences from control were plotted after normalizing a Ct value difference of 1- to 2-fold. Values represent mean ± SEM. * and ** represent significant differences P < 0.05 and P < 0.001, respectively, compared with untreated (NT) controls.
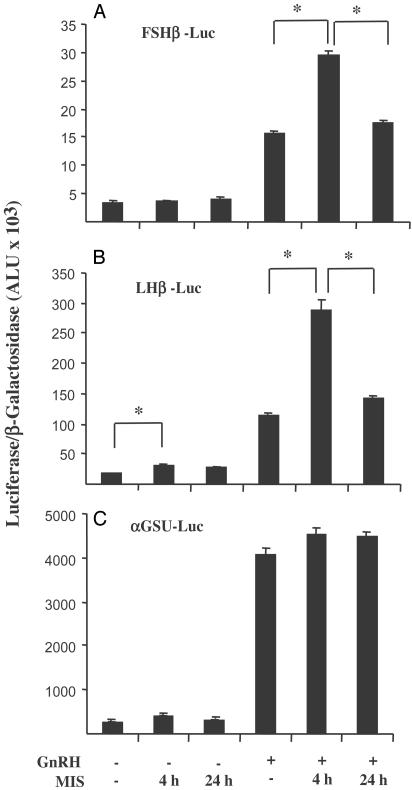
Effects of MIS on Gonadotropin Subunit Gene Transcription. To determine whether the observed effect of MIS on endogenous FSHβ mRNA levels might occur at the transcriptional level, we evaluated the effects of added MIS on transfected rat gonadotropin subunit gene promoter/luciferase reporter activity. In addition, we studied the effects of MIS in the presence or absence of GnRH, a major regulator of gonadotropin gene transcription. LβT2 cells were transfected with reporter constructs (FSHβ-Luc, LHβ-Luc, and αGSU-Luc) and stimulated with 35 nM MIS for 4 or 24 h with or without 100 nM GnRH agonist for the final 4 h. Stimulation times of 4 and 24 h were chosen because stimulation of the gonadotropin subunit gene promoter/luciferase reporters by GnRH has been shown to be maximal after 4 h (39) and because both activins and MIS affect cells optimally at or near 24 h (23, 24, 41). As shown in Fig. 3, treatment of cells transfected with FSHβ-Luc with MIS alone for either 4 or 24 h had no significant effect on luciferase activity (Fig. 3A). GnRH was able to stimulate the FSHβ promoter, as indicated by an increase in luciferase activity after exposure to 100 nM GnRH agonist for 4 h. Furthermore, despite the lack of effect of MIS alone, GnRH-stimulated FSHβ promoter activity was significantly augmented (P < 0.05) in the presence of both GnRH agonist and MIS for 4 h but was lost after 24 h of MIS treatment. Treatment with MIS for 4 h resulted in an increase in basal LHβ promoter activity, which was enhanced in the presence of GnRH (Fig. 3B). Although increased LHβ promoter activity in the absence of GnRH was still observed after 24 h treatment with MIS, synergism with GnRH was lost at this time point. Whereas GnRH treatment increased αGSU-Luc activity by 15-fold, MIS had no significant effect on either basal or GnRH-stimulated αGSU gene promoter activity (Fig. 3C). Neither MIS nor GnRH had any significant effect on the activity of the promoterless vector, pXP2, which was used as a negative control (not shown).
Fig. 3.
Effect of MIS on gonadotropin subunit gene promoter activity. Luciferase activity was measured in LβT2 cells transiently transfected with SV40-β-galactosidase and FSHβ-Luc (A), LHβ-Luc (B), and αGSU-Luc (C). Cells were stimulated for 4 or 24 h with 35 nM MIS with or without 100 nM GnRH agonist for the final 4 h. Luciferase activity was normalized to β-galactosidase activity, and results are expressed as arbitrary light units (ALU). Data points represent mean ± SEM of a representative experiment performed in triplicate. * represents a significant difference of P < 0.05 between the indicated groups.
To examine whether MIS might exert its effects on FSHβ and LHβ gene expression indirectly by affecting GnRHR expression, cells were also transfected with GnRHR-Luc and subsequently treated with MIS. No statistically significant effect of MIS on either basal or GnRH-stimulated GnRHR-Luc was observed (not shown). We also evaluated the effects of MIS on the activation of the LHβ gene promoter in αT3-1 cells, which do not express MISRII (Fig. 1). This was done to ensure that the effects of MIS on LβT2 cells were not due to a nonspecific mechanism; for example, binding to other transforming growth factor β (TGFβ) family receptors. No significant induction of LHβ gene expression was observed in this cell line (not shown).
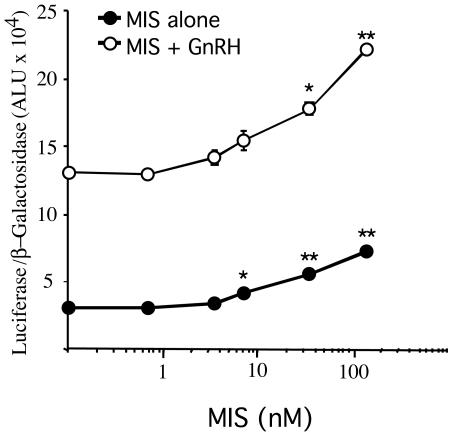
MIS Stimulates LHβ Gene Promoter Activity in a Dose-Dependent Manner. We routinely use 5 μg/ml (35 nM) of MIS in our experiments to observe complete regression of the Müllerian duct in organ culture (34) but have also observed other effects of MIS at lower concentrations (41). Because analysis of the response to MIS with GnRH indicated that MIS could increase both basal and GnRH-stimulated LHβ gene promoter activity, a dose–response experiment with MIS in the presence or absence of GnRH was performed (Fig. 4). LβT2 cells were transfected with LHβ-Luc and stimulated for 4 h with increasing concentrations of MIS, in the presence or absence of GnRH agonist. A concentration as low as 1 μg/ml MIS was sufficient to stimulate LHβ promoter activity significantly (P < 0.05), which was further increased by 5 and 20 μg/ml MIS (P < 0.01). In the absence of MIS, GnRH agonist was able to induce a 4.2-fold increase in luciferase activity. When added simultaneously, MIS was able to augment the GnRH-stimulated response further, with a significant effect of MIS again starting at a concentration of 1 μg/ml. Addition of 20 μg/ml MIS, with or without GnRH, increased LHβ promoter activity ≈2-fold over that of cells that were not treated with MIS.
Fig. 4.
MIS stimulates LHβ-Luc in a dose-dependent manner. Luciferase activity was measured in LβT2 cells transiently transfected with LHβ-Luc and SV40-β-galactosidase. Forty-four hours after transfection, cells were stimulated for 4 h with increasing concentrations of MIS alone (filled circles) or in combination with 100 nM GnRH agonist (open circles). Luciferase activity was normalized for β-galactosidase, and results are expressed as arbitrary light units (ALU). Data points represent mean ± SEM of three replicates. * and ** represent significant increases P < 0.05 and P < 0.01, respectively, from the previous point on a given curve.
Discussion
We have shown that the MISRII mRNA and protein are expressed in the rat pituitary, and that LβT2 cells, an immortalized mouse gonadotrope-derived pituitary cell line, also express MISRII mRNA, suggesting that gonadotropes may be responsive to MIS. Indeed, the addition of MIS to LβT2 cells results in a several-fold increase in endogenous FSHβ mRNA and activation of both FSHβ and LHβ promoter-driven luciferase activity, supporting a role of MIS in the regulation of gonadotropin production. Further studies of the effects of MIS in primary pituitary cell culture will be necessary to confirm these observations after careful characterization of the developmental expression of MISRII in the pituitary and the determination of the optimal time and concentration of MIS necessary.
The MISRII has been detected by in situ hybridization in the anterior pituitary of embryonic and neonatal mice of both sexes (D. Pfaff, D. T. MacLaughlin, and P. K. Donahoe, personal communication), suggesting that MIS might also affect the activity or development of the pituitary in a sexually dimorphic manner (because the high levels of serum MIS in males contrast with the lack of detectable MIS in females at those developmental stages). Perinatal expression of MISRII in the pituitary also correlates well with our studies in LβT2 cells, which have the features of mouse pituitary gonadotropes derived from embryonic day 17.5. The absence of MISRII expression in αT3-1 cells (Fig. 1 A), which have characteristics of gonadotropes derived from embryonic day 13.5 (33), is also consistent with a developmental window of MIS action on gonadotrope function.
It is probable that MIS acts as an endocrine hormone. One of the first indications of the existence of MIS was the observation that female calves of anastomosing heterosexual twins often lacked Müllerian duct derivatives, a phenomenon known as the Freemartin effect (43). As with activins (44) and bone morphogenetic proteins (BMPs) (25), one could also speculate that MIS is produced locally by the pituitary or hypothalamus, thereby increasing its local concentration and suggesting an autocrine or paracrine effect. This possibility has yet to be explored.
In the QRT-PCR studies, MIS had its greatest effect on the steady-state levels of FSHβ mRNA, but had no detectable effect on LHβ (Fig. 2), a finding that appears contrary to the results of the FSHβ and LHβ gene promoter studies (Fig. 3). This discrepancy may be explained by the relative half-lives and concentrations of the two mRNAs. Whereas the LHβ mRNA has a long half-life, the FSHβ mRNA is turned over rapidly (45), rendering its steady-state level more amenable to manipulation even by relatively modest increases in transcription. Additionally, the level of LHβ mRNA in LβT2 cells is so much higher than FSHβ mRNA that induction of its transcription by MIS might be more easily observed with reporter assays than by a change in its steady-state level. Alternatively, MIS might be regulating mRNA stability of the FSHβ mRNA, which would not be detected in the reporter assays. A trivial explanation might be that the FSHβ gene promoter fragment in the luciferase reporter construct does not include the DNA elements required for MIS-regulated expression. Unraveling the exact molecular mechanisms involved in MIS-regulated gonadotropin expression should spur exciting long-term studies and will be vital to our understanding of the role MIS plays in pituitary function and/or development.
Earlier studies have shown that immunological blockade of GnRH action prenatally resulted in enhanced postnatal MIS protein production, as measured by bioactivity in organ culture assay, and that replacement of FSH, but not LH, restored normal levels of MIS expression (27, 46). Furthermore, prenatal treatment with FSH decreased MIS expression when assayed by immunohistochemistry as well (28), although a recent report suggests otherwise postnatally (47). These results, when combined with our current observations, tempt us to speculate that an autoregulatory feedback loop exists between the pituitary, where MIS increases FSH expression, and the gonad, where FSH decreases MIS expression. It is also possible that MIS may modulate other target genes in gonadotropes that we have not yet studied, or other processes such as cell growth, differentiation, or turnover.
With the recognition that postnatal MIS expression may have physiological significance, the study of MIS has entered an exciting new era. In addition to the ability of MIS to inhibit steroidogenesis and our current hypothesis that it may also regulate gonadotrope function, recent reports that MIS can inhibit the growth and proliferation of breast, prostate, and ovarian cancer cells augur a bright future of scientific investigation for this hitherto humble hormone.
Acknowledgments
We thank Dr. Pamela Mellon for the LβT2 and αT3 cells and Drs. Nathalie di Clemente, Patricia K. Donahoe, and David T. MacLaughlin for providing unpublished information. We also thank Drs. Patricia K. Donahoe, Alan Schneyer, and Trent R. Clarke for reviewing the manuscript, David T. MacLaughlin for MIS, and Thanh Ha for assistance with Western analysis. This work was supported by the National Institute of Child Health and Human Development (NICHD)/National Institutes of Health through Cooperative Agreement U54 HD28138 as part of the Specialized Cooperative Centers Program in Reproduction Research (U.B.K. and J.T.), National Cancer Institute Grant R29-CA79459 (to J.T.), NICHD Grant R01-HD33001 (to U.B.K.), The Lalor Foundation (G.Y.B.), Reproductive Scientist Development Program K12-HD00840 through the Association of Professors of Obstetricians and Gynecologists and the National Institutes of Health (E.R.N.), and Women's Reproductive Health Research Award K12-HD01255 (to E.R.N.).
Abbreviations: MIS, Müllerian inhibiting substance; MISRII, MIS type II receptor; LH, luteinizing hormone; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; GnRHR, GnRH receptor; αGSU, glycoprotein α subunit; QRT, quantitative real-time.
References
- 1.Josso, N., Cate, R. L., Picard, J. Y., Vigier, B., di Clemente, N., Wilson, C., Imbeaud, S., Pepinsky, R. B., Guerrier, D., Boussin, L., et al. (1993) Recent Prog. Horm. Res. 48, 1–59. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira, J., Maheswaran, S. & Donahoe, P. K. (2001) Endocr. Rev. 22, 657–674. [DOI] [PubMed] [Google Scholar]
- 3.Trbovich, A. M., Sluss, P. M., Laurich, V. M., O'Neill, F. H., MacLaughlin, D. T., Donahoe, P. K. & Teixeira, J. (2001) Proc. Natl. Acad. Sci. USA 98, 3393–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durlinger, A. L., Gruijters, M. J., Kramer, P., Karels, B., Ingraham, H. A., Nachtigal, M. W., Uilenbroek, J. T., Grootegoed, J. A. & Themmen, A. P. (2002) Endocrinology 143, 1076–1084. [DOI] [PubMed] [Google Scholar]
- 5.Durlinger, A. L., Kramer, P., Karels, B., de Jong, F. H., Uilenbroek, J. T., Grootegoed, J. A. & Themmen, A. P. (1999) Endocrinology 140, 5789–5796. [DOI] [PubMed] [Google Scholar]
- 6.Segev, D. L., Hoshiya, Y., Hoshiya, M., Tran, T. T., Carey, J. L., Stephen, A. E., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2002) Proc. Natl. Acad. Sci. USA 99, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segev, D. L., Hoshiya, Y., Stephen, A. E., Hoshiya, M., Tran, T. T., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2001) J. Biol. Chem. 276, 26799–26806. [DOI] [PubMed] [Google Scholar]
- 8.Behringer, R. R., Cate, R. L., Froelick, G. J., Palmiter, R. D. & Brinster, R. L. (1990) Nature 345, 167–170. [DOI] [PubMed] [Google Scholar]
- 9.Behringer, R. R., Finegold, M. J. & Cate, R. L. (1994) Cell 79, 415–425. [DOI] [PubMed] [Google Scholar]
- 10.Mishina, Y., Rey, R., Finegold, M. J., Matzuk, M. M., Josso, N., Cate, R. L. & Behringer, R. R. (1996) Genes Dev. 10, 2577–2587. [DOI] [PubMed] [Google Scholar]
- 11.Lyet, L., Louis, F., Forest, M. G., Josso, N., Behringer, R. R. & Vigier, B. (1995) Biol. Reprod. 52, 444–454. [DOI] [PubMed] [Google Scholar]
- 12.Racine, C., Rey, R., Forest, M. G., Louis, F., Ferre, A., Huhtaniemi, I., Josso, N. & di Clemente, N. (1998) Proc. Natl. Acad. Sci. USA 95, 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouiller-Fabre, V., Carmona, S., Merhi, R. A., Cate, R., Habert, R. & Vigier, B. (1998) Endocrinology 139, 1213–1220. [DOI] [PubMed] [Google Scholar]
- 14.Teixeira, J., Fynn-Thompson, E., Payne, A. H. & Donahoe, P. K. (1999) Endocrinology 140, 4732–4738. [DOI] [PubMed] [Google Scholar]
- 15.Ueno, S., Takahashi, M., Manganaro, T. F., Ragin, R. C. & Donahoe, P. K. (1989) Endocrinology 124, 1000–1006. [DOI] [PubMed] [Google Scholar]
- 16.Baarends, W. M., Uilenbroek, J. T., Kramer, P., Hoogerbrugge, J. W., van Leeuwen, E. C., Themmen, A. P. & Grootegoed, J. A. (1995) Endocrinology 136, 4951–4962. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira, J., He, W. W., Shah, P. C., Morikawa, N., Lee, M. M., Catlin, E. A., Hudson, P. L., Wing, J., MacLaughlin, D. T. & Donahoe, P. K. (1996) Endocrinology 137, 160–165. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi, M., Hayashi, M., Manganaro, T. F. & Donahoe, P. K. (1986) Biol. Reprod. 35, 447–453. [DOI] [PubMed] [Google Scholar]
- 19.Ling, N., Ying, S. Y., Ueno, N., Shimasaki, S., Esch, F., Hotta, M. & Guillemin, R. (1986) Nature 321, 779–782. [DOI] [PubMed] [Google Scholar]
- 20.Vale, W., Rivier, J., Vaughan, J., McClintock, R., Corrigan, A., Woo, W., Karr, D. & Spiess, J. (1986) Nature 321, 776–779. [DOI] [PubMed] [Google Scholar]
- 21.DePaolo, L. V. (1997) Proc. Soc. Exp. Biol. Med. 214, 328–339. [DOI] [PubMed] [Google Scholar]
- 22.Mather, J. P., Moore, A. & Li, R. H. (1997) Proc. Soc. Exp. Biol. Med. 215, 209–222. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Vazquez, G., Kaiser, U. B., Albarracin, C. T. & Chin, W. W. (1996) Mol. Endocrinol. 10, 356–366. [DOI] [PubMed] [Google Scholar]
- 24.Pernasetti, F., Vasilyev, V. V., Rosenberg, S. B., Bailey, J. S., Huang, H. J., Miller, W. L. & Mellon, P. L. (2001) Endocrinology 142, 2284–2295. [DOI] [PubMed] [Google Scholar]
- 25.Huang, H. J., Wu, J. C., Su, P., Zhirnov, O. & Miller, W. L. (2001) Endocrinology 142, 2275–2283. [DOI] [PubMed] [Google Scholar]
- 26.Otsuka, F. & Shimasaki, S. (2002) Endocrinology 143, 4938–4941. [DOI] [PubMed] [Google Scholar]
- 27.Bercu, B. B., Morikawa, Y., Jackson, I. M. & Donahoe, P. K. (1978) Pediatr. Res. 12, 139–142. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda, T., Lee, M. M., Haqq, C. M., Powell, D. M., Manganaro, T. F. & Donahoe, P. K. (1990) Endocrinology 127, 1825–1832. [DOI] [PubMed] [Google Scholar]
- 29.Gouedard, L., Chen, Y. G., Thevenet, L., Racine, C., Borie, S., Lamarre, I., Josso, N., Massagué, J. & di Clemente, N. (2000) J. Biol. Chem. 275, 27973–27978. [DOI] [PubMed] [Google Scholar]
- 30.Clarke, T. R., Hoshiya, Y., Yi, S. E., Liu, X., Lyons, K. M. & Donahoe, P. K. (2001) Mol. Endocrinol. 15, 946–959. [DOI] [PubMed] [Google Scholar]
- 31.Visser, J. A., Olaso, R., Verhoef-Post, M., Kramer, P., Themmen, A. P. & Ingraham, H. A. (2001) Mol. Endocrinol. 15, 936–945. [DOI] [PubMed] [Google Scholar]
- 32.Turgeon, J. L., Kimura, Y., Waring, D. W. & Mellon, P. L. (1996) Mol. Endocrinol. 10, 439–450. [DOI] [PubMed] [Google Scholar]
- 33.Alarid, E. T., Windle, J. J., Whyte, D. B. & Mellon, P. L. (1996) Development (Cambridge, U.K.) 122, 3319–3329. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo, H. K., Teixeira, J., Pahlavan, N., Laurich, V. M., Donahoe, P. K. & MacLaughlin, D. T. (2002) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 766, 89–98. [DOI] [PubMed] [Google Scholar]
- 35.Ha, T. U., Segev, D. L., Barbie, D., Masiakos, P. T., Tran, T. T., Dombkowski, D., Glander, M., Clarke, T. R., Lorenzo, H. K., Donahoe, P. K. & Maheswaran, S. (2000) J. Biol. Chem. 275, 37101–37109. [DOI] [PubMed] [Google Scholar]
- 36.Ma, P. T., Gil, G., Sudhof, T. C., Bilheimer, D. W., Goldstein, J. L. & Brown, M. S. (1986) Proc. Natl. Acad. Sci. USA 83, 8370–8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jameson, J. L., Powers, A. C., Gallagher, G. D. & Habener, J. F. (1989) Mol. Endocrinol. 3, 763–772. [DOI] [PubMed] [Google Scholar]
- 38.Albarracin, C. T., Kaiser, U. B. & Chin, W. W. (1994) Endocrinology 135, 2300–2306. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser, U. B., Sabbagh, E., Katzenellenbogen, R. A., Conn, P. M. & Chin, W. W. (1995) Proc. Natl. Acad. Sci. USA 92, 12280–12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norwitz, E. R., Cardona, G. R., Jeong, K. H. & Chin, W. W. (1999) J. Biol. Chem. 274, 867–880. [DOI] [PubMed] [Google Scholar]
- 41.Laurich, V. M., Trbovich, A. M., O'Neill, F. H., Houk, C. P., Sluss, P. M., Payne, A. H., Donahoe, P. K. & Teixeira, J. (2002) Endocrinology 143, 3351–3360. [DOI] [PubMed] [Google Scholar]
- 42.di Clemente, N., Wilson, C., Faure, E., Boussin, L., Carmillo, P., Tizard, R., Picard, J. Y., Vigier, B., Josso, N. & Cate, R. (1994) Mol. Endocrinol. 8, 1006–1020. [DOI] [PubMed] [Google Scholar]
- 43.Lillie, F. (1916) Science 43, 611–613. [DOI] [PubMed] [Google Scholar]
- 44.Corrigan, A. Z., Bilezikjian, L. M., Carroll, R. S., Bald, L. N., Schmelzer, C. H., Fendly, B. M., Mason, A. J., Chin, W. W., Schwall, R. H. & Vale, W. (1991) Endocrinology 128, 1682–1684. [DOI] [PubMed] [Google Scholar]
- 45.Kilen, S. M., Szabo, M., Strasser, G. A., McAndrews, J. M., Ringstrom, S. J. & Schwartz, N. B. (1996) Endocrinology 137, 3802–3807. [DOI] [PubMed] [Google Scholar]
- 46.Bercu, B. B., Morikawa, Y., Jackson, I. M. & Donahoe, P. K. (1979) Pediatr. Res. 13, 246–249. [DOI] [PubMed] [Google Scholar]
- 47.Lukas-Croisier, C., Lasala, C., Nicaud, J., Bedecarras, P., Kumar, T. R., Dutertre, M., Matzuk, M. M., Picard, J. Y., Josso, N. & Rey, R. (2003) Mol. Endocrinol. 17, 550–561. [DOI] [PubMed] [Google Scholar]