Abstract
Division of labor is fundamental to the success of all societies. The most striking examples are the physically polymorphic worker castes in social insects with clear morphological adaptations to different roles. These polymorphic worker castes have previously been thought to be a classic example of nongentically controlled polymorphism, being mediated entirely by environmental cues. Here we show that worker caste development in the leaf-cutting ant Acromyrmex echinatior has a significant genetic component. Individuals of different patrilines within the same colony differ in their propensities to develop into minor or major workers. The mechanism appears to be plastic, with caste destiny resulting from interplay between nurture and nature. Unlike the few other recently discovered examples of a genetic influence on caste determination, the present result does not relate to any rare or exceptional circumstances, such as interspecific hybridization. The results suggest that a significant role of genetics may have been overlooked in our understanding of other complex polymorphisms of social insects.
Division of labor amongst individuals has been fundamental to the success of all societies, improving the efficiency with which tasks are carried out and thereby the fitness of the society as a whole. It is perhaps best exemplified by the extraordinary colonies of some social insects in which sterile workers exhibit extreme differences in their morphologies that are related to their specific roles (1, 2). The development of physically polymorphic worker castes is generally accepted to be controlled solely by nongenetic cues, such as nutrition and inhibitory pheromones (1–7). The effects of such environmental cues are mediated by hormones, which act at specific thresholds to cause a developmental switch involving differential gene expression (8). These act by reprogramming the critical size at which the larva begins metamorphosis or the allometric growth parameters of body parts (4). This nongenetic control of caste determination has indeed been considered to be essential to the evolution of division of labor, both in preventing the evolution of parasitic genotypes that specialize in reproduction and in avoiding colonies being genetically fixed in their caste production and unable to adapt to changing needs.
That queen caste destiny can in part be genetically controlled has been known for some years in several ant species (9–11), and was recently demonstrated in Pogonomyrmex ants (12–14). However, these intriguing results are all linked to unusual reproductive characteristics involving polymorphic queen castes or interspecific hybridization. These cases would thus appear to be merely rare exceptions to the rule of environmentally mediated caste determination. A number of studies have suggested that colonies of many ant species may differ in the size or ratios of their worker castes, and that these differences are maintained when environmental conditions are controlled (2, 15–18). These results are suggestive of genetic components being involved, but have remained inconclusive because the genetic and environmental influences could not be completely disentangled. It is now well established that honey bee workers with the same mother but different fathers (i.e., workers belonging to different patrilines within the same colony) can differ in their propensities to carry out various tasks (19–23). It has been suggested that such genetic polyethism may enable colonies to adapt their task allocation to the needs of the colony more efficiently and so may provide an explanation for the otherwise hard to explain behavior of multiple mating by queens (polyandry) (24, 25). Patriline-level comparisons provide a powerful way of separating genetic and environmental influences because nestmate workers from different patrilines have everything in common (maternal genes, environmental conditions during development) except their paternal genes. However, honey bee workers are monomorphic, and it has thus remained unclear whether such genetic influences on division of labor can be maintained once physical worker polymorphism arises.
One of the very few social insects that have been unambiguously demonstrated to have both high levels of multiple mating by queens and physically distinct worker castes are Acromyrmex leaf-cutting ants. Colonies of Acromyrmex are relatively large and long-lived, with a division of labor that is generally based on two physically distinct worker castes (26). Small workers carry out predominantly intranidal tasks, including caring for the brood, farming the fungus garden, and preventing the entry of pathogens into the colony (26–29). Large workers engage in foraging, nest maintenance and, together with a bacterial mutualist, preventing infection of the fungus garden by a specialist fungal parasite (26–28). Like other Acromyrmex species (30, 31), Acromyrmex echinatior queens are highly polyandrous (10.6 ± 1.0 matings per queen; S.S., W.O.H.H., J. S. Pedersen, and J.J.B., unpublished data).
Here we establish that there is a genetic component to worker caste polymorphism in the leaf-cutting ant A. echinatior. We first confirm that this species exhibits the same system of morphologically distinct major and minor worker castes as described above, and explicitly test whether colonies differ in the size-frequency distributions of their worker populations. We then examine the relative paternal contributions made to the two worker castes in individual colonies. By testing for differences between patrilines within colonies, we eliminate the possibility that differences could have arisen because of different rearing conditions or other environmental effects.
Materials and Methods
Colonies of A. echinatior were collected from Gamboa, Panama, in 1996 and 2001, and maintained in the laboratory under standard conditions. They were fed regularly on bramble leaves (Rubus fruticosus) placed inside plastic boxes, and were allowed to build fungus gardens within inverted plastic beakers. All colonies were monogynous.
To estimate the size-frequency distributions of the worker populations of colonies of A. echinatior, we adopted a subsampling procedure for five colonies (Ae33, 48, 132, 153, and 154). As a representative sample, we removed 20% of the fungus garden of a colony together with all of the ants that this section contained. We also collected all of the ants that were present in the foraging box and any other ants that were outside the fungus gardens. The head widths of all of the ants collected were then estimated to the nearest 0.2 mm by eye, as has been done before (32). The estimates were checked regularly with a microscope and graticule to ensure their accuracy. We compared the size-frequency distributions of ants within the fungus garden with those in the foraging box to confirm that A. echinatior showed the same system of caste-specific division of labor as other species of Acromyrmex (26). In addition, we compared the size-frequency distributions for the five colonies sampled by testing whether colonies differed in their proportions of large workers and small workers by using a G test for heterogeneity (33).
To examine the contributions that the different patrilines within a colony made to the different worker castes, we collected samples of 100 small and 100 large workers (mean ± SE: 0.84 ± 0.01-mm and 2.0 ± 0.02-mm head widths, respectively) from each of five colonies (Ae33, 48, 111, 112, and 113). These were collected from within the colony's fungus chamber on a single sampling date to control for any variation in paternity of cohorts. DNA was extracted from ant legs and amplified at four micro-satellite loci: Ech1390, Ech3385, Ech4126, and Ech4225 (34). Reactions were performed in 20-μl volumes of 1 μl of DNA, 1× reaction buffer, 0.2 mM dNTPs, 0.5 units of Taq polymerase, and 0.25, 0.35, 0.35, and 2.0 μM of the Ech1390, Ech3385, Ech4126, and Ech4225 primers, respectively. The DNA was amplified by multiplexing all four primers in Hybaid PCR Express Thermal Cyclers using a touchdown temperature program (35). This process had an initial denaturing step of 94°C for 4 min followed by two touchdown sequences of six cycles each (first sequence: 92°C for 30 s, 65.0 to 64.0°C decreasing at 0.2°C per cycle, and 72°C for 30 s; second sequence: 92°C for 45 s, 55.0 to 52.5°C decreasing at 0.5°C per cycle, and 72°C for 45 s). These were then followed by a sequence of 20 cycles in which the denaturing temperature was 92°C for 45 s, 52°C for 30 s, and 72°C for 45 s. A final elongation step of 72°C for 60 min completed the amplification process.
PCR products were run on 5% polyacrylamide gels on an ABI377 automatic sequencer (Applied Biosystems). Allele sizes were scored by comparison with internal size markers, and the genotypes of the colony queens and their multiple mates were inferred from the multilocus offspring genotypes. Where the paternities of individual workers could not be reliably determined because of their being heterozygous and having the same alleles as a heterozygous queen, the workers were excluded from the analysis. Only the five most abundant patrilines were analyzed to avoid problems of low sample sizes. We used G tests for heterogeneity to examine whether the patrilines differed from the expected ratio (the total number of small/large workers assigned to all five patrilines) in a uniform direction (33). In addition, we partitioned the G values obtained to examine which patrilines differed from the expected ratio.
Results
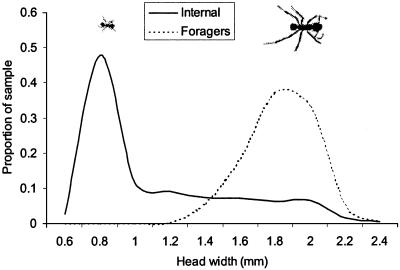
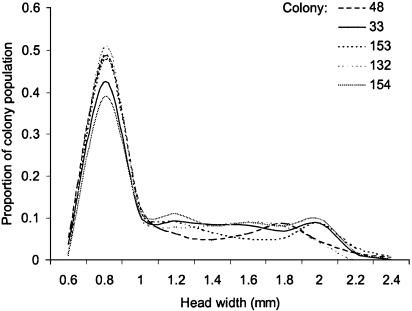
The majority of individuals found within the fungus gardens were small workers, with almost half having head widths in the 0.8 ± 0.1 mm size class (Fig. 1). By contrast, the workers sampled foraging were among the largest members of the population, having head widths of ≈1.8 mm. There were very few differences in the overall size-frequency distributions of the five colonies examined (Fig. 2), and there were no significant differences between them in the overall proportions of large workers to small workers in their colony populations (GHet = 2.62, df = 4, P > 0.05).
Fig. 1.
Size-frequency distributions for A. echinatior worker populations either located within the nest (fungus garden) or engaged in foraging. Data are smoothed lines based on the means of five colonies. See text for further details of methods.
Fig. 2.
Size-frequency distributions for total worker populations of five colonies of A. echinatior. Data are presented as smoothed lines. See text for further details of methods.
The ratios of small workers to large workers differed significantly between the patrilines for four of the five colonies examined (Ae33: GHet = 50.7, df = 4, P < 0.01; Ae48: GHet = 15.8, df = 4, P < 0.01; Ae112: GHet = 10.99, df = 3, P < 0.05; Ae113: GHet = 24.2, df = 4, P < 0.01) (Fig. 3). These results all remained significant after adjusting for multiple tests with a sequential Bonferroni correction (33). Although the fifth colony did not show any significant variation between the five most abundant patrilines (Ae111: GHet = 2.76, df = 4, P > 0.05), the two rarest of its eight patrilines each showed a strong bias toward large worker production [7 of 7 and 7 of 9 individuals; if all eight patrilines are included, then the analysis confirms there to be significant variation between patrilines in this colony as well (GHet = 16.4, df = 7, P < 0.05)]. The number of patrilines in which caste was significantly skewed toward one or other caste compared with the sample average, varied greatly from none of the most abundant patrilines in colony Ae111 to all five patrilines in colony Ae33 (Fig. 3). Several patrilines exhibited a caste skew of ≈80%, with the greatest skew being 89% (the extreme 7 of 7 large worker production in one of the rare patrilines in colony Ae111 is likely to be due to the small number of individuals sampled). However, none of the fully sampled patrilines in any colony produced only a single worker morph.
Fig. 3.
Proportion of individuals sampled per patriline for five colonies of A. echinatior (Ae33, 48, 111, 112, and 113) that were large workers (shaded) or small workers (clear). Sample sizes are given in parentheses above patriline columns together with significance levels assessed by G tests comparing small worker/large worker proportions for individual patrilines with the overall proportions for the patrilines examined (**, P < 0.01; *, P < 0.05).
Discussion
The worker populations of A. echinatior exhibit the same form of division of labor as that found in other species of Acromyrmex, with small workers specializing in intranidal tasks and foraging being carried out by large workers (26). All of the colonies examined showed significant variation in the propensities of their patrilines to develop into these large or small workers. Workers from different patrilines within a colony share the same maternal genes, are exposed to the same environmental cues, and differ only in their paternal genes. Our results therefore conclusively show that there is a significant genetic component to the development of polymorphism in the worker caste of A. echinatior. The honey bee was previously the only social insect in which such a genetic influence on the division of labor between workers had been conclusively shown, and it had appeared that such an influence was impossible in species where physically distinct worker castes had evolved. The present results show that this is not the case and that genetic variation for caste predisposition, and thus division of labor, has been maintained in Acromyrmex ants.
Importantly, all of the patrilines that were fully sampled produced at least a small proportion of both castes. Individuals of a particular patriline are therefore not entirely restricted to developing into a single caste. Rather, they appear to be predisposed to develop into a particular caste, but are still capable of developing into the other caste as well, and in some cases will do so. Although this could be caused by the influence of maternal genes, it does suggest that the genetic control of worker caste in A. echinatior may be plastic rather than hardwired. Further support for the mechanism being plastic comes from the lack of significant differences between colonies in the size-frequency distributions of their total worker populations. Even given the high mating frequencies of the queens, it might be expected that, under a hardwired system of genetic control, significant differences between colonies would emerge. However, the differences that existed between colonies were negligible, with all colonies showing approximately the same (presumably optimum) distribution. In addition, genetic factors alone cannot completely explain morphological worker caste determination, because changes in caste ratios with colony age, and possibly in response to environmental stimuli, are known to occur in Acromyrmex (27), and other polymorphic ant species (2, 36–38). A combination of genetic and environmental effects would therefore seem to be necessary.
The results are strikingly similar to those found previously for task allocation in honey bees. Honey bee colonies are similar in population size to Acromyrmex ants, but their division of labor is based on monomorphic workers differing in their response thresholds for particular tasks (23, 39). Like Acromyrmex ants, honey bees are highly polyandrous (40), and numerous studies have demonstrated that worker patrilines differ in their propensities to carry out particular tasks (19–23). This results in a genetically mediated division of labor that is plastic in operation and is thought to be caused by patrilines differing in their response thresholds to task-related environmental stimuli. A particular honey bee worker genotype may have a propensity to engage in a particular task because it has a relatively low response threshold for the relevant stimuli. However, it may still engage in other tasks if the stimuli levels are below this threshold. Similarly, workers of other genotypes may also engage in the task if the colony experiences a greater need for it or lacks a genotype with a high probability to perform it. It appears likely that caste determination in Acromyrmex ants may operate in a similar manner. The growth of ant larvae is affected by environmental cues such as nutrition and pheromones (2, 4–7), and it thus seems likely that it is the response thresholds of the larvae to these cues that are genetically determined. For example, if large workers produce pheromones that inhibit larvae from developing into the same caste (6), then larvae of genotypes with high response thresholds to the pheromones will have a greater propensity to become large workers. More detailed studies will be required to establish whether this is in fact the mechanism, and to determine when during the developmental process the switch between large worker and small worker occurs. A plastic system based on an interaction between genotype and environmental cues would allow the changes in caste ratios with colony age or in response to changes in environmental stimuli that have been recorded in some ant species (2, 36–38). Such a system could also explain the findings of differences in caste ratios between colonies or between queens within polygynous colonies (2, 15–18), suggesting that a genetic influence on caste determination may be the case in many ants and other social insects.
It has been suggested that a genetic influence on division of labor may give genetically diverse social insect colonies greater flexibility in the allocation of workers to particular tasks, which may allow them to respond to changing needs quicker and more effectively (24). This hypothesis has gained empirical support from studies on the flexibility of division of labor in honey bee colonies (23, 39, 41, 42) and may apply in the same way in leaf-cutting ants. Having different groups of brood responding to caste-determining factors at different threshold levels may be more flexible than if all brood were to respond at the same level. This may make the colony better able to produce the most appropriate caste allocation of brood and allow the caste ratios to be altered in accordance with changing needs more rapidly.
Our finding of a genetic influence on caste determination in leaf-cutting ants demonstrates the important roles of both nurture and nature in the development of complex polymorphisms. It also has significant implications for our understanding of the evolution of female multiple mating (polyandry) in social insects. Polyandry is hard to explain because it is associated with substantial costs, but clear benefits have proved hard to establish (43, 44). One of the main hypotheses to explain the evolution of polyandry in the social insects is that it improves the division of labor of their colonies through intracolonial genetic polyethism (24, 25). Until this study, evidence supporting the theory has only been found in monomorphic honey bee workers and, under the previous assumption of nongenetically determined castes, it could not have applied to species with polymorphic workers. That it occurs as well in the polymorphic leaf-cutting ants lends considerable weight to it as an explanation for the evolution of polyandry in these phenomenally successful insects.
Acknowledgments
We thank Allen Herre and The Smithsonian Tropical Research Institute for providing facilities in Gamboa for the collection of the ant colonies, and the Instituto Nacional de Recursos Naturales Renovables for permission to collect and export them from Panama to Denmark. We are also grateful to Sylvia Mathiasen for outstanding technical assistance and to Jane Stout, David Nash, Duur Aanen, Boris Baer, and the anonymous referees for comments on the manuscript. This research has been supported by European Union Marie Curie Fellowship Contract HPMFCT-2000-00543 (to W.O.H.H.) and by the European Union Research Training Network “INSECTS” under Contract HPRN-CT-2000-00052 (S.S., S.V.B., and J.J.B.).
References
- 1.Oster, G. F. & Wilson, E. O. (1978) Caste and Ecology in the Social Insects, Monographs in Population Biology (Princeton Univ. Press, Princeton), No. 12. [PubMed]
- 2.Hölldobler, B. & Wilson, E. O. (1990) The Ants (Belknap Press, Cambridge, MA).
- 3.Huxley, J. S. (1932) Problems of Relative Growth (Dover, New York).
- 4.Wheeler, D. E. (1991) Am. Nat. 138, 1218–1238. [Google Scholar]
- 5.Nijhout, H. F. & Wheeler, D. E. (1982) Am. Nat. 57, 109–133. [Google Scholar]
- 6.Wheeler, D. E. & Nijhout, H. F. (1984) J. Insect Physiol. 30, 127–135. [Google Scholar]
- 7.Brian, M. V. (1979) in Social Insects, ed. Hermann, H. R. (Academic, New York), Vol. 1, pp. 121–222. [Google Scholar]
- 8.Evans, J. D. & Wheeler, D. E. (2001) BioEssays 23, 62–68. [DOI] [PubMed] [Google Scholar]
- 9.Winter, U. & Buschinger, A. (1986) Entomol. General. 11, 125–127. [Google Scholar]
- 10.Heinze, J. & Buschinger, A. (1989) Insectes Soc. 36, 139–155. [Google Scholar]
- 11.Fersch, R., Buschinger, A. & Heinze, J. (2000) Insectes Soc. 47, 280–284. [Google Scholar]
- 12.Volny, V. P. & Gordon, D. M. (2002) Proc. Natl. Acad. Sci. USA 99, 6108–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Julian, G. E., Fewell, J. H., Gadau, J., Johnson, R. A. & Larrabee, D. (2002) Proc. Natl. Acad. Sci. USA 99, 8157–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahan, S. H., Parker, J. D., Rissing, S. W., Johnson, R. A., Polony, T. S., Weiser, M. D. & Smith, D. R. (2002) Proc. R. Soc. London Ser. B 269, 1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston, A. B. & Wilson, E. O. (1985) Ann. Entomol. Soc. Am. 78, 8–11. [Google Scholar]
- 16.Billick, I. (2002) Oecologia 132, 244–249. [DOI] [PubMed] [Google Scholar]
- 17.Breed, M. D. (2002) Insectes Soc. 49, 125–128. [Google Scholar]
- 18.Fraser, V. S., Kaufmann, B., Oldroyd, B. P. & Crozier, R. H. (2000) Behav. Ecol. Sociobiol. 47, 188–194. [Google Scholar]
- 19.Robinson, G. E. & Page, R. E. (1988) Nature 333, 356–358. [Google Scholar]
- 20.Frumhoff, P. C. & Baker, J. (1988) Nature 333, 358–361. [Google Scholar]
- 21.Page, R. E., Robinson, G. E. & Fodrk, M. K. (1989) Nature 338, 576–579. [Google Scholar]
- 22.Oldroyd, B. P., Sylvester, A., Wongsiri, S. & Rinderer, T. E. (1994) Behav. Ecol. Sociobiol. 34, 25–30. [Google Scholar]
- 23.Robinson, G. E. (1992) Annu. Rev. Entomol. 37, 637–665. [DOI] [PubMed] [Google Scholar]
- 24.Crozier, R. H. & Page, R. E. (1985) Behav. Ecol. Sociobiol. 18, 105–115. [Google Scholar]
- 25.Page, R. E., Robinson, G. E., Fondrk, M. K. & Nasr, M. E. (1995) Behav. Ecol. Sociobiol. 36, 387–396. [Google Scholar]
- 26.Wetterer, J. K. (1999) Sociobiology 34, 119–144. [Google Scholar]
- 27.Weber, N. A. (1972) Mem. Am. Philos. Soc. 92, 1–146. [Google Scholar]
- 28.Poulsen, M. P., Bot, A. N. M., Currie, C. R. & Boomsma, J. J. (2002) Insectes Soc. 49, 15–19. [Google Scholar]
- 29.Hughes, W. O. H., Eilenberg, J. & Boomsma, J. J. (2002) Proc. R. Soc. London Ser. B 269, 1811–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boomsma, J. J., Fjerdingstad, E. J. & Frydenberg, J. (1999) Proc. R. Soc. London Ser. B 266, 249–254. [Google Scholar]
- 31.Ortius-Lechner, D., Maile, R., Morgan, E. D., Petersen, H. C. & Boomsma, J. J. (2003) Insectes Soc. 50, 113–119. [Google Scholar]
- 32.Wilson, E. O. (1980) Behav. Ecol. Sociobiol. 7, 143–156. [Google Scholar]
- 33.Sokal, R. R. & Rohlf, F. J. (1995) Biometry (Freeman, New York).
- 34.Ortius-Lechner, D., Gertsch, P. J. & Boomsma, J. J. (2000) Mol. Ecol. 9, 107–118. [DOI] [PubMed] [Google Scholar]
- 35.Don, R. H., Cox, P. T., Wainwright, B. J., Baker, K. & Mattick, J. S. (1991) Nucleic Acids Res. 19, 4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passera, L., Roncin, E., Kaufmann, B. & Keller, L. (1996) Nature 379, 630–631. [Google Scholar]
- 37.Wilson, E. O. (1983) Behav. Ecol. Sociobiol. 14, 55–60. [Google Scholar]
- 38.McGlynn, T. P. & Owen, J. P. (2002) Insectes Soc. 49, 8–14. [Google Scholar]
- 39.Page, R. E. & Erber, J. (2002) Naturwissenschaften 89, 91–106. [DOI] [PubMed] [Google Scholar]
- 40.Palmer, K. A. & Oldroyd, B. P. (2000) Apidologie 31, 235–248. [Google Scholar]
- 41.Fewell, J. H. & Bertram, S. M. (1999) Behav. Ecol. Sociobiol. 45, 171–179. [Google Scholar]
- 42.Fewell, J. H. & Page, R. E. (2000) Behav. Ecol. Sociobiol. 48, 173–181. [Google Scholar]
- 43.Boomsma, J. J. & Ratnieks, F. L. W. (1996) Philos. Trans. R. Soc. London B 351, 947–975. [Google Scholar]
- 44.Simmons, L. W. (2001) Sperm Competition and Its Evolutionary Consequences in Insects (Princeton Univ. Press, Princeton).