Abstract
Base pairing between the 5′ end of U7 small nuclear RNA (snRNA) and the histone downstream element (HDE) in replication-dependent histone pre-mRNAs is the key event in 3′-end processing that leads to generation of mature histone mRNAs. We have cloned the Drosophila U7 snRNA and demonstrated that it is required for histone pre-mRNA 3′-end processing in a Drosophila nuclear extract. The 71-nt Drosophila U7 snRNA is encoded by a single gene that is embedded in the direct orientation in an intron of the Eip63E gene. The U7 snRNA gene contains conserved promoter elements typical of other Drosophila snRNA genes, and the coding sequence is followed by a 3′ box indicating that the Drosophila U7 snRNA gene is an independent transcription unit. Drosophila U7 snRNA contains a trimethyl-guanosine cap at the 5′ end and a putative Sm-binding site similar to the unique Sm-binding site found in other U7 snRNAs. Drosophila U7 snRNA is ≈10 nt longer than mammalian U7 snRNAs because of an extended 5′ sequence and has only a limited potential to form a stem-loop structure near the 3′ end. The extended 5′ end of Drosophila U7 snRNA can base pair with the HDE in all five Drosophila histone pre-mRNAs. Blocking the 5′ end of the U7 snRNA with a complementary oligonucleotide specifically blocks processing of a Drosophila histone pre-mRNA. Changes in the HDE that abolish or decrease processing efficiency result in a reduced ability to recruit U7 snRNA to the pre-mRNA.
Replication-dependent histone pre-mRNAs are processed at the 3′ end by an endonucleolytic cleavage that occurs between two conserved sequence elements located within 100 nt 3′ of the stop codon: a highly conserved stem–loop structure and a purine-rich histone downstream element (HDE). The stem–loop structure, consisting of a 6-bp stem and a 4-nt loop, is recognized by a stem–loop-binding protein (SLBP) (1), also designated hairpin-binding protein (2), whereas the HDE is recognized by U7 small nuclear ribonucleoprotein (snRNP). Known mammalian U7 snRNPs contain an ≈60-nt U7 snRNA (3, 4) as well as common Sm proteins and at least one unique Sm-like protein, Lsm 10, that replaces D1 and D2 Sm proteins present in other snRNPs (5). In HeLa cells, a fraction of cellular U7 snRNP is found associated with an unique zinc finger protein, hZFP100 (6). Interaction of U7 snRNP with histone pre-mRNA primarily occurs through base pairing between the 5′ end of U7 snRNA and the HDE (7, 8). In mammalian cells U7 snRNP is relatively rare, ≈1,000 times less abundant than major spliceosomal snRNPs.
U7 snRNPs are quantitatively localized to Cajal bodies in Xenopus oocytes (9, 10) and mammalian cells (11). In addition, a subset of the Cajal bodies are localized near histone genes (12, 13), suggesting a possible role for the Cajal bodies in histone mRNA biosynthesis. Cajal bodies have only been detected in vertebrates, and orthologs of the protein coilin are not obvious in the Drosophila or Caenorhabditis elegans genomes.
Drosophila histone pre-mRNAs are processed at the 3′ end by a mechanism similar to that in mammalian cells (14). The stem-loop is recognized by Drosophila SLBP (dSLBP) (15), which resembles mammalian SLBP only within the unique RNA-binding domain. Removal of dSLBP from a Drosophila nuclear extract abolishes 3′-end processing of histone pre-mRNA (14). Moreover, loss-of-function mutations in the dSLBP gene result in reduction in the efficiency of correct 3′-end processing of histone pre-mRNAs in vivo and accumulation of histone mRNAs that are polyadenylated downstream from the stem-loop (16).
Drosophila histone pre-mRNAs contain a stretch of purines downstream from the 3′-processing site that resembles the HDE in mammalian and sea urchin pre-mRNAs. Mutations in this purine-rich element result in disruption of 3′-end processing in vitro, suggesting that this sequence interacts with a Drosophila counterpart of the mammalian U7 snRNP (14). In addition, treatment of a Drosophila nuclear extract with anti-Sm Ab results in a significant reduction of processing activity (14). Although these studies support the role of a putative U7 snRNP in Drosophila 3′-end processing, the lack of any obvious orthologs of Lsm 10 and hZFP 100, two U7-specific human proteins, in the Drosophila genome raised the possibility that histone pre-mRNA 3′-end processing in Drosophila differs from processing in mammalian cells and may use an alternative, U7-independent mechanism. Here we describe cloning of Drosophila U7 snRNA and demonstrate that it is required for 3′-end processing of histone pre-mRNAs.
Materials and Methods
Histone Pre-mRNA Substrates and Processing. The dH3* pre-mRNA and the conditions for 3′-end processing have been described (14). The 2′-O-methyl oligonucleotides complementary to the 5′ end of U7 snRNAs had the following sequences (5′–3′): AAAGAACUGUAACACUU (anti-mouse U7) and GAAUAAAAAUUUUCAAU (anti-Drosophila U7). Both oligonucleotides were synthesized by Dharmacon (Lafayette, CO).
Formation and Isolation of Processing Complexes. One microgram of the 105-nt dH3* pre-mRNA was incubated in 100 μl of buffer D (20 mM Hepes-potassium hydroxide, pH 7.9/100 mM KCl/0.5 mM DTT/0.2 mM EDTA, pH 8/20% glycerol) for 15 min at room temperature with 5-fold molar excess of a 2′-O-methyl adaptor oligonucleotide (CGAGCUCGAAUUCGCCC, Dharmacon) that was complementary to the first 17 nt of the pre-mRNA and contained biotin at the 3′ end. The annealed RNAs were added to a processing reaction containing 1.0 ml of sterile water, 0.5 ml of 200 mM EDTA (pH 8), 1.0 ml of buffer D, and 2.5 ml of a nuclear extract from Drosophila Kc cells. The reaction was incubated for 5 min in a water bath at room temperature, cooled on ice, and then rotated for 2 h at 4°C with 50 μl of streptavidin agarose beads (Sigma). The beads were washed several times with buffer D, and the bound RNA was recovered by phenol extraction and ethanol precipitation, separated in an 8% polyacrylamide denaturing gel, and detected by silver staining. A small portion of the beads was resuspended in SDS-sample buffer and the bound proteins resolved in a 15% SDS-polyacrylamide gel.
Isolation of Drosophila U7 snRNA. Y12 mouse mAb against Sm proteins (1 ml) was adjusted to 100 mM Tris (pH 8) and rotated with 50 μl of protein G-Sepharose beads (Pierce) for 2 h at 4°C. The beads were collected and rotated for 2 h at 4°C with 1 ml of a nuclear extract from Drosophila Kc cells. The RNA bound to the beads was recovered by phenol extraction and ethanol precipitation, separated in an 8% polyacrylamide denaturing gel, and visualized by staining with ethidium bromide. Drosophila U7 snRNA was excised and recovered from the gel slice by electroelution.
Cloning of Drosophila U7 snRNA. Approximately 1 ng of the electroeluted Drosophila U7 snRNA was polyadenylated at the 3′ end by 2 units of poly(A) polymerase from Escherichia coli (Ambion). The cDNA was synthesized in the presence of 30 ng of U7T oligonucleotide (GCAAGCTTCGGATCCTTTTTTTTTTTTTTTTTT) and 1 μl of ImProm-II reverse transcriptase (Promega), according to the manufacturer's protocol and extended at the 3′ end with deoxyadenosines by using 30 units of terminal deoxynucleotidyl transferase (Invitrogen), 1 mM dATP, and reaction buffer provided by the manufacturer. A small portion of the cDNA was amplified by AmpliTaq DNA polymerase (Applied Biosystems) by using the U7T oligonucleotide as both the forward and reverse primer. PCR products were cloned by using TOPO TA cloning kit (Invitrogen) and clones with appropriately sized inserts sequenced in the University of North Carolina DNA sequencing facility.
Anti-U7 Probe and Northern Blotting. The 71-nt cDNA for Drosophila U7 snRNA was cloned into pGEM3 in reverse orientation downstream from the T7 promoter and transcribed by T7 RNA polymerase in the presence of [α-32P]UTP (25 μCi), 10 nM unlabeled UTP, and 100 nM remaining NTPs. RNA for Northern blotting was prepared as described (17).
Immunoprecipitation of Drosophila U7 snRNA with Anti-2,2,7-Trimethyl Guanosine (TMG) Ab. RNA isolated from 50 μl of a Drosophila Kc cell nuclear extract was incubated for 2 h at 4°C with 12.5 μg of αTMG Ab-1 mAb (Oncogene) and absorbed on 40 μl of protein G-Sepharose beads (Amersham Pharmacia). The RNA was recovered from the beads by phenol extraction, precipitated in the presence of 10 μg of glycogen and a 32P-labeled 86-nt RNA to control for the efficiency of precipitation, resolved in an 8% denaturing polyacrylamide gel, and analyzed by Northern blotting.
Results
Identification of a Putative Drosophila U7 snRNA. U7 snRNAs were initially identified in sea urchins (18, 19) and subsequently in vertebrates, but have not been isolated from other metazoans. The importance of a purine-rich downstream element in processing of Drosophila histone pre-mRNAs and the reduction in processing activity caused by depletion of a nuclear extract with anti-Sm Ab strongly suggested that U7 snRNP is a component of Drosophila3′-end processing machinery (14). Identification of U7 snRNA in the Drosophila genome database by using similarity to known U7 snRNAs was not possible because of their small size and lack of sequence conservation.
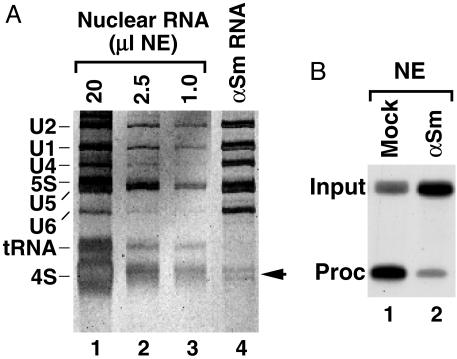
To identify the putative Drosophila U7 snRNA, we immunoprecipitated anti-Sm reactive snRNPs, separated the precipitated RNA in a denaturing gel, and looked for low abundance snRNAs by using silver staining. The anti-Sm Ab precipitated the snRNAs involved in pre-mRNA splicing and two smaller RNA species (Fig. 1A, lane 4, and Fig. 2B, lane 1). In total nuclear RNA, these two bands were masked by large amounts of 4S RNA (Fig. 1 A, lanes 1–3). The size of the two RNA species, ≈70 nt, and their precipitability by the anti-Sm Ab made them primary candidates for Drosophila U7 snRNA. No RNA was precipitated by a nonspecific mAb (Fig. 2B, lane 2). Mock depletion of the Drosophila nuclear extract with the nonspecific Ab had no effect on processing whereas depletion with the anti-Sm Ab significantly reduced processing efficiency (Fig. 1B, lanes 1 and 2), suggesting that the putative Drosophila U7 snRNP was partially removed by this Ab.
Fig. 1.
An Sm-precipitable factor is necessary for efficient Drosophila pre-mRNA processing. (A) Total RNA from decreasing amounts of a Drosophila nuclear extract (lanes 1–3) and the RNA species precipitated by anti-Sm Ab (lane 4) were resolved in a denaturing polyacrylamide gel and detected by silver staining. The arrow indicates the position of a putative Drosophila U7 snRNA. (B) In vitro processing of Drosophila-specific dH3* pre-mRNA in a Kc nuclear extract preincubated with a nonspecific mAb (mock, lane 1) or with anti-Sm Ab (αSm, lane 2).
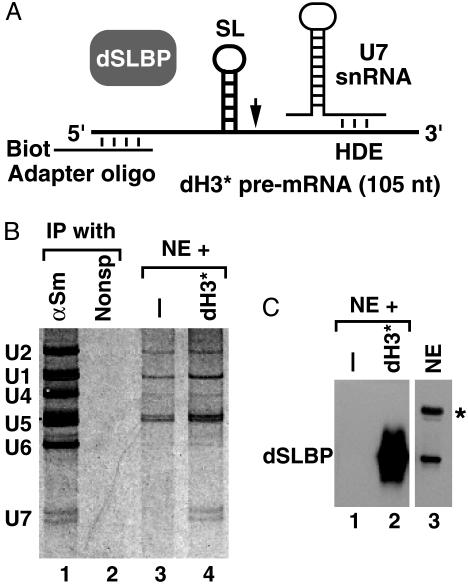
Fig. 2.
Identification of a putative Drosophila U7 snRNA. (A) A scheme for formation of processing complexes on a Drosophila-specific dH3* pre-mRNA (thick line). (B)A Drosophila Kc nuclear extract was incubated in the presence (lane 4) or absence (lane 3) of the WT dH3* pre-mRNA. Bound RNAs were recovered from 90% of the streptavidin beads, separated in an 8% polyacrylamide gel, and detected by silver staining. Lanes 1 and 2 contain RNA precipitated from the Kc nuclear extract by using anti-Sm (αSm) or a nonspecific mAb, respectively. The pre-mRNA substrate annealed to the 2′-O-methyl adapter oligonuclotide remained in the organic phase during phenol extraction. (C) The remaining portion of each processing reaction (10%) was boiled in SDS-loading dye, and bound proteins were analyzed by Western blotting for the presence of dSLBP by using anti-dSLBP Ab (lanes 1 and 2). Lane 3 contains an aliquot of the nuclear extract used for formation of processing complexes. The star indicates a cross-reacting protein present in Drosophila nuclear extracts.
To determine whether the two RNAs were involved in 3′-end processing, we purified processing complexes and analyzed their RNA content. Previously we showed that processing complexes containing U7 snRNP can be assembled on synthetic pre-mRNAs in mammalian nuclear extracts (17). A large processing reaction was prepared by using a Drosophila Kc nuclear extract and a 105-nt Drosophila-specific dH3* pre-mRNA preannealed to a short 2′-O-methyl oligonucleotide (the adaptor oligonucleotide) complementary to the first 17-nt of the pre-mRNA. This adaptor oligonucleotide contained biotin on the 3′ end and formed a strong duplex with the pre-mRNA thus allowing subsequent isolation of processing complexes on streptavidin beads (Fig. 2 A). The processing reaction was briefly incubated at room temperature to facilitate formation of processing complexes. We also prepared a control sample containing only the adaptor oligonucleotide and no pre-mRNA to determine the background of RNAs nonspecifically bound to streptavidin agarose. The RNA collected on streptavidin beads was separated in a denaturing gel and detected by silver staining. The two short RNA species initially detected in anti-Sm precipitates were also present in the processing complexes but were absent from the control sample (Fig. 2B, lanes 3 and 4), suggesting that they represent RNA species involved in histone pre-mRNA processing. In both the control sample and the sample containing pre-mRNA, there were small amounts of spliceosomal snRNAs and 5S RNA nonspecifically bound to steptavidin beads.
The processing complexes were also analyzed for the presence of dSLBP, a known component of 3′-end processing machinery. A small fraction of streptavidin beads (≈10%) containing bound processing complexes was analyzed by gel electrophoresis and Western blotting by using anti-dSLBP. The dH3* pre-mRNA bound large amounts of dSLBP, and no dSLBP was detected in a control sample lacking any pre-mRNA (Fig. 2C, lanes 1 and 2).
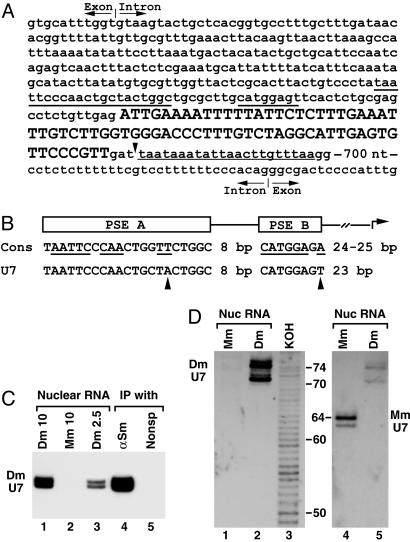
Cloning of Drosophila U7 snRNA. The two putative Drosophila U7 snRNAs were gel purified from anti-Sm precipitates and polyadenylated at the 3′ end by using E. coli poly(A) polymerase followed by synthesis of a cDNA. The cDNA was extended at the 3′ end by addition of deoxyadenosines by using terminal transferase, amplified by PCR, and cloned. One clone contained a sequence of 71 nt, which had the properties expected for the Drosophila U7 snRNA. This 71-nt sequence (GenBank accession no. AY310908) is located on chromosome 3L within a 1,104-nt intron of the gene encoding ecdysone-induced protein 63E, Eip63E. The Eip63E gene spans ≈93 kb and has been cytologically mapped to 63E3–63E5.
Alignment of 13 Drosophila genes encoding U1, U2, and U4 snRNAs revealed the presence of two conserved elements in the promoter region of these genes: a 21-nt proximal sequence element A (PSE A) and an 8-nt proximal element B (PSE B) located 8-nt downstream (20, 21). Transcription begins ≈25-nt downstream from the end of PSE B. The same conserved elements are present in Drosophila genes encoding the U5 snRNA (J. P. Jin and W.F.M., unpublished results). Both the PSE A and PSE B can be readily identified upstream of the 71-nt Drosophila cDNA (Fig. 3A). When compared to the consensus sequence of the spliceosomal snRNA genes, each promoter element of the putative U7 snRNA gene contains a single nucleotide substitution (Fig. 3B). The putative transcription start site, corresponding to the first nucleotide of the isolated cDNA, is located 23 nt 3′ of the last nucleotide of the PSE B. Downstream of the U7 snRNA-coding region there is an AT-rich region closely resembling the termination site found in other RNA Pol II-specific genes for Drosophila snRNAs (Fig. 3A). We conclude that the Drosophila U7 snRNA gene has features typical of other snRNA genes transcribed by RNA polymerase II.
Fig. 3.
Cloning and detection of Drosophila U7 snRNA. (A) The gene for Drosophila U7 snRNA is nested in the intron of the Eip63E gene. The cloned 71-nt cDNA of Drosophila U7 snRNA is shown in uppercase letters and the sequence retrieved from the Drosophila genome is shown in lowercase letters. The 71-nt sequence is followed by GAT, likely included in the longer form of the U7 snRNA. The putative 3′-end signal is underlined with the thin line. Underlined with the thick lines are the two promoter elements, PSE A and B. A part of the intronic sequences surrounding the U7 gene and both the 5′-splice site (top) and the 3′-splice site (bottom) are shown. (B) Comparison of the consensus sequence for the PSE A and B promoter elements found in Drosophila genes for U1, U2, and U4 snRNAs (Cons) with the Drosophila U7 snRNA gene. Conserved nucleotides are underlined, and the nucleotides in the U7 promoter that differ from the consensus are indicated with arrowheads. (C) RNA isolated from 10 or 2.5 μlofa Drosophila nuclear extract (lanes 1 and 3), 10 μl of a mouse nuclear extract (lane 2), or RNA precipitated from 50 μlof the Drosophila nuclear extract by either anti-Sm Ab (lane 4) or a control mAb (lane 5) were resolved in 8% denaturing gels and analyzed by Northern blotting by using the anti-Drosophila U7 probe. (D) Nuclear RNA from mouse myeloma cells (lanes 1 and 4) or Drosophila Kc cells (lanes 2 and 5) was resolved in a high-resolution denaturing gel and analyzed by Northern using the anti-Drosophila U7 probe (Left) followed by hybridization with the anti-mouse U7 probe (Right). Lane 3 contains a ladder of RNA fragments differing in length by 1 nt generated from a 5′-end-labeled 86-nt RNA by partial potassium hydroxide hydrolysis (not shown in Right). The numbers indicate the length of RNA fragments in nucleotides.
We hybridized an antisense Drosophila U7 probe to Drosophila nuclear RNA and anti-Sm precipitated RNA. The probe detected the same two bands previously identified by silver staining as a putative U7 snRNA (Fig. 3C, lanes 1 and 3) and no cross-reacting RNA species were detected in mouse nuclear RNA (Fig. 3C, lane 2). The relative intensities of the two bands detected by silver staining and hybridization were equivalent, suggesting that they represent two forms of the same RNA. The two RNAs were enriched in the anti-Sm precipitate and completely absent from an RNA preparation precipitated by a control mAb (Fig. 3C, lanes 4 and 5).
To precisely determine the size of the two Drosophila U7 snRNA species, we separated Drosophila and mouse total nuclear RNA preparations in a high-resolution denaturing gel next to products of partial potassium hydroxide hydrolysis of a 5′-end-labeled 86-nt RNA. The species-specific U7 snRNAs were detected by Northern blotting by using two consecutive hybridizations, first with the probe complementary to the Drosophila U7 snRNA (Fig. 3D Left) and then with the anti-mouse U7 snRNA probe (Fig. 3D Right). The lower Drosophila U7 snRNA band comigrated with the 71-nt product of partial RNA hydrolysis whereas the upper U7 snRNA comigrated with a 74-nt hydrolysis product. Based on this result, we conclude that the 71-nt cDNA represents the shorter U7 snRNA and that the longer U7 snRNA contains three additional nucleotides, likely at the 3′ end (Fig. 3A). In agreement with the published sequence (3), the mouse U7 snRNA was 64-nt long, significantly shorter than the two Drosophila U7 snRNAs.
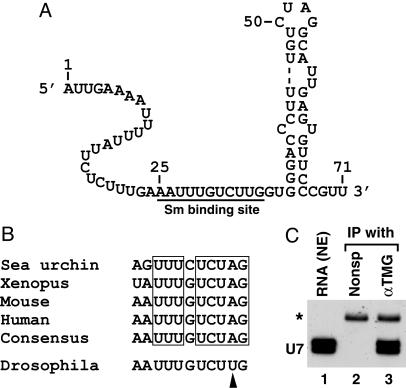
Features of the Drosophila U7 snRNA. One of the characteristic features shared by mammalian, Xenopus, and sea urchin U7 snRNAs is ability of the 3′ end of the RNA to fold into a stable stem–loop structure (22). Surprisingly, Drosophila U7 snRNA is predicted to form a relatively unstable secondary structure at the 3′ end, containing a 2-nt bulge and an internal loop with the longest double-stranded region containing 5 bp, one of which is a GU bp (Fig. 4A).
Fig. 4.
Features of Drosophila U7 snRNA. (A) The sequence and a possible secondary structure of 71-nt Drosophila U7 snRNA. The putative Sm-binding site is underlined. (B) Comparison of the Sm-binding site from four previously identified U7 snRNAs and from Drosophila U7 snRNA. The consensus sequence for the previously identified U7 snRNAs is shown and the invariant nucleotides in the U7 snRNA of all four species are boxed. The arrowhead indicates the nucleotide in Drosophila Sm-binding site that departs from the consensus. (C) RNA extracted from a Kc nuclear extract (lane 1) was incubated with either a nonspecific mAb (lane 2) or αTMG Ab (lane 3), and the RNA was recovered from protein G-Sepharose and analyzed by Northern blotting. A small amount of 32P-labeled 86-nt RNA (asterisk) was added to each sample before ethanol precipitation to control for the efficiency of RNA recovery.
The Sm-binding site in the sea urchin and vertebrate U7 snRNAs, present immediately upstream of the stem–loop, differs from that found in spliceosomal snRNAs (23). This atypical Sm-binding site (Fig. 4B) is in part responsible for the low abundance of the U7 snRNA in the nucleus (23) and for the unusual composition of U7 snRNP, which lacks D1 and D2 Sm core proteins and contains instead an Sm-like protein, Lsm 10 (5). In Drosophila U7 snRNA, 25 nt from the 5′ end there is an adenosine, which begins a stretch of nucleotides with a nearly perfect match to the U7 Sm-binding site consensus (Fig. 4B). Thus, the unusual Sm-binding site in U7 snRNAs has also been conserved in Drosophila.
Known U7 snRNAs are synthesized by the RNA polymerase II and contain the TMG cap. To determine whether Drosophila U7 snRNA contains the same cap, we precipitated nuclear RNA with the Ab-1 mAb directed against the TMG cap. The Ab-1 mAb precipitated Drosophila U7 snRNA that was readily detectable by Northern blotting (Fig. 4C, lane 3) whereas the nonspecific Ab was unable to precipitate any U7 snRNA (Fig. 4C, lane 2). Analysis of a larger sample of immunoprecipitated RNA by silver staining revealed that the anti-TMG Ab in addition to the U7 snRNA precipitated all spliceosomal snRNAs (not shown). We conclude that Drosophila U7 snRNA is modified at the 5′ end with the TMG structure.
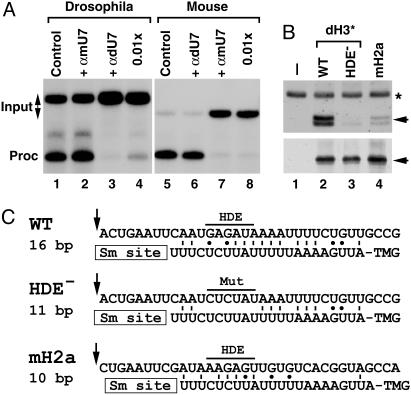
Drosophila U7 snRNA Is Required for 3′-End Processing. Binding of U7 snRNP to histone pre-mRNA is critical for 3′-end processing and occurs primarily via base pairing between the 5′ end of U7 snRNA and the HDE. Blocking the U7 5′ end by a complementary antisense 2′-O-methyl oligonucleotide (24, 25) or oligodeoxynucleotide-mediated degradation of the 5′ end by RNase H (4, 26) is a very potent and specific method of inhibiting histone pre-mRNA processing. To demonstrate that the cloned Drosophila U7 snRNA is involved in histone pre-mRNA processing, we designed a 2′-O-methyl oligonucleotide complementary to the first 17 nt of the U7 snRNA and determined its ability to affect the processing of the dH3* pre-mRNA. The efficiency of processing of the dH3* pre-mRNA was unchanged in the presence of 1.0 μg of the anti-mouse U7 oligonucleotide (αmU7) used as a negative control (Fig. 5A, lanes 1 and 2). However, processing was abolished in the presence of the same amount of the anti-Drosophila U7 oligonucleotide (αdU7) and reduced by 95% in the presence of 10 ng of this oligonucleotide (Fig. 5A, lanes 3 and 4). Processing of the mouse H2a-614 pre-mRNA was specifically abolished by both 1.0 μg and 10 ng of the anti-mouse U7 oligonucleotide (Fig. 5A, lanes 7 and 8) but was not affected by 1.0 μgofthe Drosophila-specific oligonucleotide (Fig. 5A, lane 6). These results indicate that the specific oligonucleotide, directed to either Drosophila or mouse U7 snRNA, is capable of selectively inhibiting processing in the species-specific nuclear extract but not in the heterologous nuclear extract.
Fig. 5.
Drosophila U7 snRNA is present in histone pre-mRNA processing complexes. (A) In vitro processing of Drosophila dH3* (lanes 1–4) and mouse H2a-614 pre-mRNAs (lanes 5–8) in a Drosophila and mouse nuclear extract, respectively. Processing was carried out in the absence of any oligonucleotide (lanes 1 and 5) or in the presence of 2′-O-methyl oligonucleotides complementary to first 17 nt of Drosophila U7 snRNA (αdU7, lanes 3, 4, and 6) or mouse U7 snRNA (αmU7, lanes 2, 7, and 8). The oligonucleotides were used in the processing reaction at either 100 ng/μl (lanes 2, 3, 6, and 7) or 1 ng/μl (lanes 4 and 7). (B)A Drosophila nuclear extract was incubated in the absence of exogenous pre-mRNA (lane 1) or in the presence of three pre-mRNAs preannealed with the biotinylated adapter oligonucleotide: the WT Drosophila dH3*, its mutant containing 6-nt substitutions within the core HDE sequence (HDE–), and the mouse H2a-614 pre-mRNA. Bound Drosophila U7 snRNA and dSLBP, each indicated with the arrow, were analyzed by Northern blotting (Upper) and Western blotting (Lower). A small amount of 32P-labeled 86-nt RNA (asterisk) was added to each sample analyzed by Northern blotting. (C) A potential base pairing between the 5′ end of Drosophila U7 snRNA and the downstream element in three different pre-mRNAs analyzed in Fig. 5B. The arrow indicates a putative cleavage site located 4 nt after the stem (14) and the core purine-rich HDE beginning 13 nt after the cleavage site is overlined. The GU bp is indicated with the dot.
To determine whether the U7 snRNA is present in processing complexes, we assembled processing complexes in a Kc nuclear extract by using three different substrates: efficiently processed WT dH3* pre-mRNA, the HDE– mutant containing a 6-nt mutation of the purine-rich HDE previously shown to abolish processing (14), and the mouse H2a-614 pre-mRNA, which is processed very inefficiently in Drosophila nuclear extracts (14). Processing complexes were isolated on streptavidin beads by using the antisense biotinylated RNA annealed to the 5′ end of each pre-mRNA (Fig. 2 A). A portion of the processing complexes was tested by Western blotting for the presence of dSLBP (Fig. 5B Lower) and the remainder by Northern blotting for the presence of U7 snRNA (Fig. 5B Upper). In the absence of pre-mRNA, no dSLBP or U7 snRNA was bound to the beads (Fig. 5B, lane 1). The three pre-mRNAs bound similar amounts of dSLBP because they all contain the same stem–loop structure (Fig. 5B Lower, lanes 2–4). Both species of Drosophila U7 snRNA were readily detectable in the complexes assembled on the WT dH3* pre-mRNA (Fig. 5B Upper, lane 2). This pre-mRNA can potentially form a 16-bp duplex with Drosophila U7 snRNA (Fig. 5C). Only a trace amount of U7 snRNA was bound to the HDE– pre-mRNA, 7-fold less than the amount bound to the WT pre-mRNA (Fig. 5B Upper, lane 3), although it could still form 11 bp with the U7 snRNA, most of which are downstream from the purine-rich HDE sequence (Fig. 5C). The mouse H2a-614 pre-mRNA bound ≈3.5-fold less U7 snRNA than did the WT Drosophila substrate (Fig. 5B Upper, lane 4). This pre-mRNA can form only 10 bp with the U7 snRNA and these are mostly clustered within the purine-rich HDE core (Fig. 5C). These data indicate that the cloned U7 snRNA is a component of the Drosophila-processing complexes and the amount of the U7 snRNP that associates with the pre-mRNA corresponds to the efficiency of its 3′-end processing.
Discussion
U7 snRNP is a minor snRNP consisting of a short TMG-capped RNA and both common Sm proteins as well as a number of mostly unknown U7-specific proteins (27). Interaction of the U7 snRNP with the HDE is facilitated by SLBP (17) and dictates the site of cleavage of the pre-mRNA at a fixed distance from the HDE (28, 29). These studies strongly suggest that U7 snRNP forms a nucleation center for binding other processing factors, including the endonuclease that ultimately results in cleavage of histone pre-mRNA. Identifying protein components of this key processing factor will provide critical clues about the molecular mechanisms responsible for 3′-end processing of histone pre-mRNAs. Here we describe cloning of Drosophila U7 snRNA as an important step in studying the detailed events in 3′-end processing of histone pre-mRNAs in a genetically tractable system.
Drosophila U7 snRNA is encoded by a genomic sequence that is embedded in a direct orientation within an intron of the Eip63E gene. The U7 snRNA-coding sequence is preceded by readily recognizable promoter elements, PSE A and B, highly conserved in all Drosophila snRNA genes transcribed by RNA Pol II and is followed by a typical, AT-rich termination site. It is likely that the two substitutions of the conserved nucleotides present in the promoter of the U7 snRNA gene decrease the efficiency of transcription from the gene and contribute to the low abundance of U7 snRNA compared to the spliceosomal snRNAs. This presence of both the promoter and termination sequences indicates that U7 snRNA is transcribed independently of the Eip63E gene rather than being generated by processing of the excised intron, as in the case of many small nucleolar RNAs (30). Generation of U7 snRNA by independent transcription is also strongly supported by the presence of the TMG cap structure at the 5′ end of U7 snRNA. The phenomenon of nested genes that are transcribed in the same orientation, although rare, has been reported for other genes in Drosophila (31). To our knowledge this is the first example of an snRNA gene located in the intron of another gene.
One of the features shared by all known U7 snRNAs is a unique Sm-binding site, which differs from the Sm-binding site found in spliceosomal snRNAs and is essential for structural and functional characteristics of U7 snRNP. Mutating this U7-specific Sm-binding site to a sequence matching the spliceosomal consensus Sm-binding site results in production of defective particles that lack the U7-specific Lsm 10 protein (5) and that are unable to support histone pre-mRNA processing in vivo (23). Drosophila U7 snRNA contains a well conserved U7-specific Sm-binding site that differs from that in mammalian U7 snRNAs by a substitution of only 1 nt. Single nucleotide differences in the sequence of the Sm-binding site were previously observed among mammalian, sea urchin, and Xenopus U7 snRNAs, and those differences most likely involve less conserved positions. The presence of a conserved Sm site in the Drosophila U7 snRNA suggests that the Drosophila genome encodes an ortholog of the Lsm 10 protein (5), although this cannot be recognized by sequence comparison.
Although the sea urchin and vertebrate U7 snRNAs end in a relatively long stem–loop, the 3′ end of the Drosophila U7 snRNA has the potential to form a rather unstable and short secondary structure. Because the stem–loop structure is not conserved at the sequence level (32), its only function may be to provide stability against 3′ exonucleases in the cell. Thus, the lack of a stable 3′-terminal secondary structure in Drosophila U7 snRNA might be compensated by a specific protein–RNA interaction that would efficiently protect its 3′ end.
Drosophila U7 snRNA exists in a 71- and a 74-nt form, and both forms can assemble into U7 snRNP active in histone pre-mRNA processing. Drosophila U7 snRNA is 10–15 nt longer than known U7 snRNAs, including mammalian U7 snRNAs, which are 63-nt long (3, 4), and Xenopus and sea urchin U7 snRNAs, which each contain <60 nt (32, 33). This difference in length results from the unusually long, 24-nt 5′ region in Drosophila U7 snRNAs, located between the TMG cap and the Sm-binding site. The 5′ region is exceptionally AU-rich, containing only two cytidines and two guanosines.
In Drosophila, all of the replication-dependent histone genes are located in a single tandemly repeated unit. As a result there are only five different HDEs in this organism, one for each class of the histone pre-mRNA (14). The 5′ region of Drosophila U7 snRNA can form with all five Drosophila HDEs extensive duplexes, containing 14–18 bp (not shown). Although multiple alternative alignments of the U7 snRNP with each HDE can be proposed, it is likely that a single alignment is strongly preferred, given the role of U7 snRNP in determining the site of cleavage at a specific distance from the HDE (29). In the mouse and human genomes, there are ≈70 nonallelic histone genes, each containing a different sequence within the HDE (34). Mammalian U7 snRNAs also contain a relatively long 5′ end, allowing sufficient base pairing with the variable HDEs in these organisms. In contrast, the 5′-end region of sea urchin U7 snRNAs is much shorter than that in mammalian and Drosophila U7 snRNAs and consists of only 8–9 nt but forms a perfect duplex with the highly conserved purine-rich HDE, which is present in all sea urchin histone pre-mRNAs (32).
The human and mouse genomes contain only a single active gene for U7 snRNA (35); although multiple U7 snRNA genes, which differ slightly from each other within the coding region, are present in sea urchins and Xenopus (32, 33). There are no other sequences closely related to the cloned U7 snRNA in the Drosophila genome. This, together with the extensive base pairing potential between each of the five Drosophila histone pre-mRNAs and the cloned U7 snRNA, suggests that Drosophila contains only a single U7 snRNA gene.
Acknowledgments
We thank J. P. Jin and H. Kalckar for help in bioinformatics, E. Wagner for comments on the manuscript, S. Whitfield for help in preparing illustrations, and M. Adams (University of North Carolina, Chapel Hill) and D. Rio (University of California, Berkeley) for frozen stocks of Drosophila Kc cells. This work was supported by National Institutes of Health Grant GM58921 (to W.F.M.).
Abbreviations: HDE, histone downstream element; dSLBP, Drosophila stem–loop-binding protein; PSE, proximal sequence element; TMG, 2,2,7-trimethyl guanosine; sn, small nuclear; RNP, ribonucleoprotein.
References
- 1.Wang, Z.-F., Whitfield, M. L., Ingledue, T. I., Dominski, Z. & Marzluff, W. F. (1996) Genes Dev. 10, 3028–3040. [DOI] [PubMed] [Google Scholar]
- 2.Martin, F., Schaller, A., Eglite, S., Schümperli, D. & Müller, B. (1997) EMBO J. 16, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soldati, D. & Schümperli, D. (1988) Mol. Cell. Biol. 8, 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mowry, K. L. & Steitz, J. A. (1987) Science 238, 1682–1687. [DOI] [PubMed] [Google Scholar]
- 5.Pillai, R. S., Will, C. L., Lührmann, R., Schümperli, D. & Müller, B. (2001) EMBO J. 20, 5470–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominski, Z., Erkmann, J. A., Yang, X., Sanchez, R. & Marzluff, W. F. (2002) Genes Dev. 16, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaufele, F., Gilmartin, G. M., Bannwarth, W. & Birnstiel, M. L. (1986) Nature 323, 777–781. [DOI] [PubMed] [Google Scholar]
- 8.Bond, U. M., Yario, T. A. & Steitz, J. A. (1991) Genes Dev. 5, 1709–1722. [DOI] [PubMed] [Google Scholar]
- 9.Wu, C.-H. H. & Gall, J. G. (1993) Proc. Natl. Acad. Sci. USA 90, 6257–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu, C. H., Murphy, C. & Gall, J. G. (1996) RNA 2, 811–823. [PMC free article] [PubMed] [Google Scholar]
- 11.Gall, J. G. (2000) Annu. Rev. Cell Dev. Biol. 16, 273–300. [DOI] [PubMed] [Google Scholar]
- 12.Shopland, L. S., Byron, M., Stein, J. L., Lian, J. B., Stein, G. S. & Lawrence, J. B. (2001) Mol. Biol. Cell 12, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey, M. R. & Matera, A. G. (1995) Proc. Natl. Acad. Sci. USA 92, 5915–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominski, Z., Yang, X., Raska, C. S., Santiago, C. S., Borchers, C. H., Duronio, R. J. & Marzluff, W. F. (2002) Mol. Cell. Biol. 22, 6648–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan, E., Santiago, C., Parker, E. D., Dominski, Z., Yang, X., Lanzotti, D. J., Ingledue, T. C., Marzluff, W. F. & Duronio, R. J. (2001) Genes Dev. 15, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanzotti, D. J., Kaygun, H., Yang, X., Duronio, R. J. & Marzluff, W. F. (2002) Mol. Cell. Biol. 22, 2267–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominski, Z., Zheng, L.-X., Sanchez, R. & Marzluff, W. F. (1999) Mol. Cell. Biol. 19, 3561–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galli, G., Hofstetter, H., Stunnenberg, H. G. & Birnstiel, M. L. (1983) Cell 34, 823–828. [DOI] [PubMed] [Google Scholar]
- 19.Strub, K., Galli, G., Busslinger, M. & Birnstiel, M. L. (1984) EMBO J. 3, 2801–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen, R. C., Wang, Y., Hardin, S. B. & Stumph, W. E. (1998) Nucleic Acids Res. 26, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNamara-Schroeder, K. J., Hennessey, R. F., Harding, G. A., Jensen, R. C. & Stumph, W. E. (2001) J. Biol. Chem. 276, 31786–31792. [DOI] [PubMed] [Google Scholar]
- 22.Dominski, Z. & Marzluff, W. F. (1999) Gene 239, 1–14. [DOI] [PubMed] [Google Scholar]
- 23.Grimm, C., Stefanovic, B. & Schümperli, D. (1993) EMBO J. 12, 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotten, M., Oberhauser, B., Brunar, H., Holzner, A., Issakides, G., Noe, C. R., Schaffner, G., Wagner, E. & Birnstiel, M. L. (1991) Nucleic Acids Res. 19, 2629–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominski, Z., Sumerel, J., Hanson, R. J. & Marzluff, W. F. (1995) RNA 1, 915–923. [PMC free article] [PubMed] [Google Scholar]
- 26.Cotten, M., Schaffner, G. & Birnstiel, M. L. (1989) Mol. Cell. Biol. 9, 4479–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, H. O., Tabiti, K., Schaffner, G., Soldati, D., Albrecht, U. & Birnstiel, M. L. (1991) Proc. Natl. Acad. Sci. USA 88, 9784–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharl, E. C. & Steitz, J. A. (1996) Proc. Natl. Acad. Sci. USA 93, 14659–14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharl, E. C. & Steitz, J. A. (1994) EMBO J. 13, 2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filipowicz, W. & Pogacic, V. (2002) Curr. Opin. Cell Biol. 14, 319–327. [DOI] [PubMed] [Google Scholar]
- 31.Misra, S., Crosby, M. A., Mungall, C. J., Matthews, B. B., Campbell, K. S., Hradecky, P., Huang, Y., Kaminker, J. S., Millburn, G. H., Prochnik, S. E., et al. (2002) Genome Biol. 3, 0083.1–0083.22. [Google Scholar]
- 32.Birnstiel, M. L. & Schaufele, F. J. (1988) in Structure and Function of Major and Minor Small Ribonucleoprotein Particles, ed. Birnstiel, M. L. (Springer, Berlin), pp. 155–182.
- 33.Phillips, S. C. & Birnstiel, M. L. (1992) Biochim. Biophys. Acta Gene Struct. Expression 1131, 95–98. [DOI] [PubMed] [Google Scholar]
- 34.Marzluff, W. F., Gongidi, P., Woods, K. R., Jin, J. P. & Maltais, L. (2002) Genomics 80, 487–498. [PubMed] [Google Scholar]
- 35.Gruber, A., Soldati, D., Burri, M. & Schümperli, D. (1991) Biochim. Biophys. Acta Gene Struct. Expression 1088, 151–154. [DOI] [PubMed] [Google Scholar]