Abstract
Effector T cell responses can be modulated by competing positive or negative signals transduced by natural killer (NK) cell receptors. This raises the possibility that dominant T cell stimulation might promote autoimmune reactions. In rheumatoid arthritis (RA), the severity of autoimmune and inflammatory joint disease correlates with large numbers of CD4+CD28– T cells, which are scarce in healthy individuals. For poorly defined reasons, these T cells are autoreactive, implying that they may contribute to disease manifestations. CD4+CD28– T cells in peripheral blood and synovial tissue of RA patients were found to express NKG2D, a costimulatory receptor that is absent on normal CD4 T cells. NKG2D was induced by tumor necrosis factor α and IL-15, which are abundant in inflamed synovia and RA patient sera. RA synoviocytes aberrantly expressed the stress-inducible MIC ligands of NKG2D, which stimulated autologous CD4+CD28– T cell cytokine and proliferative responses. Peripheral blood serum samples of RA patients contained substantial amounts of synoviocyte-derived soluble MICA, which failed to induce down-modulation of NKG2D because of the opposing activity of tumor necrosis factor α and IL-15. These results suggest that a profound dysregulation of NKG2D and its MIC ligands may cause autoreactive T cell stimulation, thus promoting the self-perpetuating pathology in RA and possibly other autoimmune diseases.
Maintaining effective immune surveillance without provoking autoimmune reactions requires the precise titration of effector T cell responses. This fine-tuning may involve the integration of negative or positive signals transduced by inhibitory or activating isoforms of the killer cell Ig-like receptors (KIR), which interact with MHC class I HLA-A, -B, or -C alleles and the inhibitory CD94-NKG2A and activating CD94-NKG2C heterodimers, which interact with HLA-E (1, 2). Some of these receptors have the capacity to modulate thresholds of T cell antigen receptor-dependent T cell activation (1, 2). For example, CD8 T cells express inhibitory KIR or CD94-NKG2A receptors after persistent antigen-driven stimulation, which down-modulate effector responses in chronic infections and malignancies but may safeguard against autoimmune reactions (3–5). By contrast, the role of activating KIR isoforms and CD94-NKG2C in T cell modulation is less clear, mainly because they are usually coexpressed with their inhibitory counterparts, which have higher ligand affinities and thus convey dominant negative signals (1, 2). However, in the rare absence of inhibitory receptors, the activating isoforms may augment T cell effector functions and contribute to autoimmune pathology (6, 7). This is corroborated by the association of disease severity in rheumatoid arthritis (RA) with expression of the activating KIR2DS2 receptor by autoreactive CD4+CD28– T cells in individuals with proper HLA-C ligand alleles (7).
An activating receptor lacking an apparent antagonist is NKG2D, which interacts with the MHC class I-related MICA and MICB glycoproteins among other ligands (8). These have no role in antigen presentation, have a restricted tissue distribution in intestinal epithelium, and can be stress-induced in permissive types of cells by viral and bacterial infections, malignant transformation, and proliferation (9–14). NKG2D is a C-type lectin-like activating receptor that signals through the associated DAP10 adaptor protein similar to CD28 (15). It is expressed on most natural killer (NK) cells, CD8 T cells, and γδ T cells, but not on CD4 T cells (8). Ligand engagement of NKG2D activates NK cells and potently costimulates effector T cells (8, 12, 13). However, expression of NKG2D is controlled by ligand-induced down-modulation, which is transient and rapidly reversed in the presence of IL-15 (16, 17).
Because ligand binding unconditionally triggers NKG2D, its dysregulation together with anomalous expression of MIC in local tissue environments could promote autoreactive T cell stimulation. We explored this possibility in the context of the pathology of RA, which involves lymphocyte infiltrates, inflammatory mediators, and synovial hyperplasia due to aggressive proliferation of fibroblast-like synoviocytes and macrophages (18, 19). Prognoses of joint erosions and disease severity correlate with high frequencies of clonally expanded CD4+CD28– T cells, which are rare in healthy individuals but occur in other autoimmune disorders (7, 20–24). These T cells can be cytotoxic, secrete large amounts of IFN-γ, and proliferate upon stimulation with autologous adherent mononuclear cells (21, 25). Although this aggregate evidence is insufficient to directly implicate CD4+CD28– T cells in autoimmunity in RA, their expansion and unusual properties suggest some involvement in this disease. The present results show that large proportions of RA CD4+CD28– T cells express NKG2D, which stimulates autoreactive responses against RA synoviocytes displaying anomalous expression of MIC.
Materials and Methods
Peripheral Blood Samples, Tissue Materials, and Cell Preparations. Peripheral blood was obtained from 30 unrelated patients fulfilling the 1988 American College of Rheumatology criteria for RA and from 20 random healthy volunteers. Synovial tissues were obtained from 19 RA and 2 osteoarthritis patients at the time of joint arthroplasty or by closed-needle synovial biopsy. Five peripheral blood and synovial tissue samples were matched; the remainder were from different patient populations. These activities were approved by local institutional review boards, and all subjects gave written informed consent. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque density gradient centrifugation. CD4 T cells were purified from unseparated peripheral blood by negative selection using a RosetteSep (StemCell Technologies, Vancouver) enrichment mixture. NKG2D– CD4 T cells were isolated from purified CD4 T cell populations with a FACSVantage cell sorter (BD Biosciences, San Diego) after immunofluorescence staining with anti-NKG2D mAb 1D11 (8) and phycoerythrin-goat anti-mouse Ig F(ab′)2. For isolation of synovial cells, tissues were minced, partially digested with 0.3 mg/ml collagenase (Sigma), pressed through a metal screen, and centrifuged through Ficoll–Hypaque.
Flow Cytometry and Immunohistochemistry. PBMC, synovial mononuclear cells, and unseparated synoviocyte suspensions were examined by two- or three-color flow cytometry using various combinations of anti-CD3, -CD4, -CD8, -CD56, -TCRαβ, -CD28, -CD45RA, or -CD45RO (BD PharMingen) conjugated to phycoerythrin, FITC, or PerCP. Binding of anti-NKG2D and anti-Ki-67 (BD PharMingen) mAbs was detected with phycoerythrin- or FITC-goat anti-mouse Ig F(ab′)2. Biotinylated anti-MIC mAb 6D4 (10) was detected with streptavidin-FITC. For intracellular staining, cells were permeabilized with 0.1% saponin for 10 min at 4°C before antibody exposure. For immunohistochemistry staining, 4-μm cryostat sections were made from synovial tissues embedded in OCT compound (Sakura Fine Technologies, Tokyo) and snap-frozen in liquid nitrogen. Sections were fixed in acetone, air dried, rehydrated in TBS, and blocked sequentially with 0.03% hydrogen peroxide, 25% normal goat serum, and 25% pooled human serum, all in TBS. Sections were incubated with anti-MIC mAb 6D4, anti-NKG2D mAb 1D11, or isotype-matched IgG for 1 h at room temperature in a humid chamber. Antibody binding was detected by using biotinylated secondary IgG and streptavidin-horseradish peroxidase (DAKO). Sections were counterstained with Harris' hematoxylin and mounted with Glycergel (DAKO).
Induction of NKG2D and Generation of T Cell Clones and Synovial Fibroblast Cell Lines. PBMC from healthy volunteers and purified CD4+NKG2D– T cells from RA patients were cultured in RPMI medium 1640, 10% FCS, and antibiotics with or without IL-15 (15 ng/ml), tumor necrosis factor α (TNF-α) (15 ng/ml), IL-10 (20 ng/ml), IL-12 (20 ng/ml), or IFN-γ (10 ng/ml) (R & D Systems) for up to 10 days. T cells were tested for NKG2D expression before and at various time points after cytokine exposure by flow cytometry. In some experiments, CD4 T cells were stimulated with solid-phase anti-CD3 (OKT3, 50 ng/ml; Orthobiotech, Raritan, NJ). For generation of T cell clones, CD4+CD28–NKG2D+ T cells were sorted from RA PBMC and synovial cell suspensions and seeded at 0.5 cells per well in 96-well round-bottom microtiter plates by using a FACSVantage cell sorter. T cells were cultured with weekly restimulations with γ-irradiated allogeneic PBMC (105 cells per well) in RPMI medium 1640 supplemented with 8% FCS, 2% pooled human serum, antibiotics, and IL-2 (5 units/ml; Chiron). RA synovial fibroblast cultures were established from cell suspensions prepared from two biopsies (see above) by adherence to tissue culture plates followed by removal of nonadherent cells. Adherent cells were cultured in DMEM supplemented with 10% FCS, 1 mM nonessential amino acids, 1 mM sodium pyruvate, and antibiotics. After four passages, cultures were free of contaminating mononuclear cells and expressed high levels of MIC as confirmed by flow cytometry.
RNA Blot Hybridization. Total cellular RNA was extracted and purified from freshly isolated CD4 T cells and CD4 T cells cultured in the presence of cytokines by using STAT-60 reagent (Tel-Test, Friendswood, TX). Standard procedures were followed for gel electrophoresis and blot hybridization.
Cytotoxicity, Cytokine Release, and T Cell Proliferation Assays. T cell cytolytic activity was tested in standard 4-h 51Cr release assays with labeled target cells that included the mouse mastocytoma P815 cell line for redirected lysis and MICA transfectants of the B-lymphoblastoid C1R cell line (9). Redirected lysis was tested in the presence of anti-NKG2D and anti-CD3 (OKT3) mAbs or isotype controls, each at a concentration of 2 μg/ml. Assays were done in triplicate, and results were scored according to the standard formula. In the cytokine release assays, resting (14 days after stimulation) T cells (105 per well) were stimulated with either solid-phase anti-CD3 with or without anti-NKG2D or control Ig as described (13) or with equal numbers of autologous or mismatched irradiated synovial fibroblasts. For blocking experiments, effector or stimulator cells were incubated with saturating amounts of anti-NKG2D, anti-MIC (mAb 6D4), or control IgG 30 min before and throughout the coculture. After 24 h, T cell supernatants were collected from triplicate wells and pooled. Secreted IFN-γ and TNF-α were quantitated by commercial ELISA with matched antibody pairs in relation to standard pairs (R & D Systems). T cell proliferation was measured with resting T cells (105 cells per well) after activation with solid-phase mAb as described above. Cultures were pulsed with [3H]thymidine on day 3 and collected after 12 h by using a micromate cell harvester (Packard). Incorporated radioactivity was determined by using Uni-Filter GF/C plates (Packard) and a TopCount liquid scintillation counter (Packard).
ELISA of Soluble MICA and Modulation of NKG2D. Five serum samples matched with MIC-positive synovial biopsies and five unmatched serum samples from RA patients were tested for the presence of soluble MICA by ELISA as described (17). Modulation of NKG2D on peripheral blood CD4 T cells among PBMC from RA patients by soluble MIC containing RA sera (1:5 dilutions of sera) in the presence or absence of neutralizing mAb against IL-15 (0.5 μg/ml) and TNF-α (0.2 μg/ml; R & D Systems) was examined after 24 h of incubation by staining with anti-CD4 and anti-NKG2D and flow cytometry (17). As a control experiment, T cells were exposed to the soluble MIC+ BT 450–85 serum from a breast cancer patient, which down-modulates NKG2D on CD8 T cells (17). The amounts of IL-15 and TNF-α in patient sera were determined by commercial ELISA with matched antibody pairs in relation to standard pairs (R & D Systems).
Results and Discussion
CD4+CD28– T Cells from RA Patients Express NKG2D. Peripheral blood lymphocytes (PBL) from 30 RA patients and 20 healthy volunteers were profiled for NKG2D expression by antibody staining and flow cytometry. The amounts and distribution of NKG2D among RA CD8 T cells, NK cells, and γδ T cells were similar to those recorded with the control PBL (data not shown; ref. 8). However, 11–61% (mean 18%) of RA CD4 T cells were positive for NKG2D, whereas nearly all control CD4 T cells were negative (Fig. 1 A and B) (8). We examined whether NKG2D expression was associated with CD4+CD28– T cells by multicolor flow cytometry. Consistent with previous observations, these T cells occurred among all RA but not normal PBL, at frequencies ranging from 12% to 50% (mean 15%) (21, 22, 24). With all RA PBL samples, NKG2D was preferentially expressed on CD4+CD28– T cells, with positive cell numbers ranging from 35% to 100% (mean 47%). By contrast, NKG2D was present on 3–36% (mean 8%) of CD4+CD28+ T cells (representative data are shown in Fig. 1 C and D). In four cases, large expansions of CD4+CD28– T cells (28–50%) correlated with disproportionally higher numbers of NKG2D+ cells (80–100%), suggesting an involvement of NKG2D in T cell proliferation. Altogether, however, there was no significant relationship between the proportion of these T cells and the frequency of expression of NKG2D.
Fig. 1.
Preferential expression of NKG2D on the CD4+CD28– T cell subset in RA patients. (A and B) Three-color flow cytometry shows that CD4+ T cells among normal PBL are negative for NKG2D, whereas significant proportions (25% in this representative sample) from RA patients are positive. (C and D) Among gated CD4+ T cells from RA PBL, NKG2D expression is preferentially associated with the CD28– subset. The sample numbers tested are indicated in the text. (E and F) This association is confirmed with freshly isolated CD4+CD28– synovial T lymphocytes (STL). The results shown are representative of five samples. Numbers in dot plots indicate percentages of cells in quadrants.
RA CD4+CD28– T cells also occur at sites of tissue injury, including synovial joints and rheumatoid vasculitis (7, 21). As with circulating RA CD4 T cells, NKG2D was present on synovial tissue CD4 T cells, preferentially on those lacking CD28 (Fig. 1 E and F), whereas its expression on other lymphocyte infiltrates was unchanged (data not shown). Thus, circulating and resident CD4 T cells from patients with RA frequently expressed NKG2D. Its main occurrence among the autoreactive CD28– subset suggests that it may participate in tissue destruction. NKG2D was also present on variable proportions of RA CD4+CD28+ T cells and was associated with a memory phenotype as indicated by CD45 isotype expression (Fig. 1 C–F and data not shown).
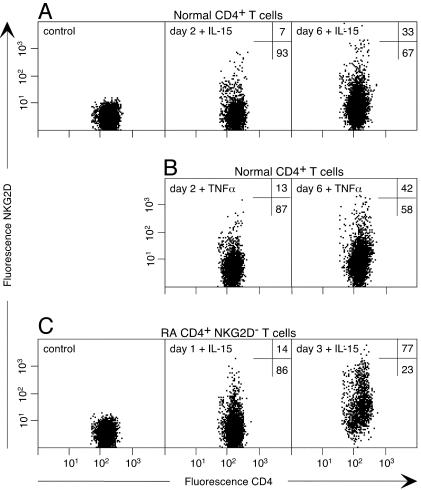
Induction of NKG2D on CD4 T Cells by IL-15 and TNF-α. Under normal conditions, the tissue distribution of the MIC ligands of NKG2D is limited to intestinal epithelium where intraepithelial CD8 T cells have diminished expression of NKG2D as a result of ligand-induced down-modulation (9, 16, 17). However, NKG2D can be up-regulated on these T cells by IL-15 (16), which is prominent among the proinflammatory cytokines that are abundant in RA synovia (26–28). We tested whether IL-15 might be responsible for the aberrant expression of NKG2D on RA CD4 T cells. Normal PBL were cultured in the presence or absence of IL-15 for several days, and surface NKG2D on lymphocyte subsets was monitored by flow cytometry. With CD8 T cells and NK cells, IL-15 had no effect on NKG2D expressed at maximum levels (data not shown). However, NKG2D was progressively induced on CD4 T cells, with small positive populations (5–10% of CD4 T cells) appearing as early as 48 h after addition of IL-15 (Fig. 2A). Maximum induction was reached after 6–7 days of culture, with ≈30–40% of CD4 T cells expressing NKG2D (Fig. 2 A). Thereafter, NKG2D decreased gradually unless the culture was replenished with fresh IL-15. A similar but markedly accelerated induction of NKG2D was observed with sorted RA CD4+NKG2D– T cells. Already after 24 h, 10–20% of the T cells expressed NKG2D, and the majority was positive after 3 days (Fig. 2C). As indicated by intracellular staining of permeabilized cells, the more rapid appearance of surface NKG2D was likely due to redistribution of intracellular protein in a subpopulation of the RA CD4+NKG2D– T cells (data not shown), whereas the delayed response was due to induction of mRNA (Fig. 3).
Fig. 2.
Induction of NKG2D on CD4 T cells by IL-15 and TNF-α. (A and B) Exposure of normal PBL to IL-15 or TNF-α results in induced expression of NKG2D on gated CD3+CD4+ T cells. (C) This induction is markedly accelerated with CD4+NKG2D– T cells sorted from RA PBL. Data for TNF-α not shown. Numbers in dot plots indicate percentages of NKG2D positive and negative cells.
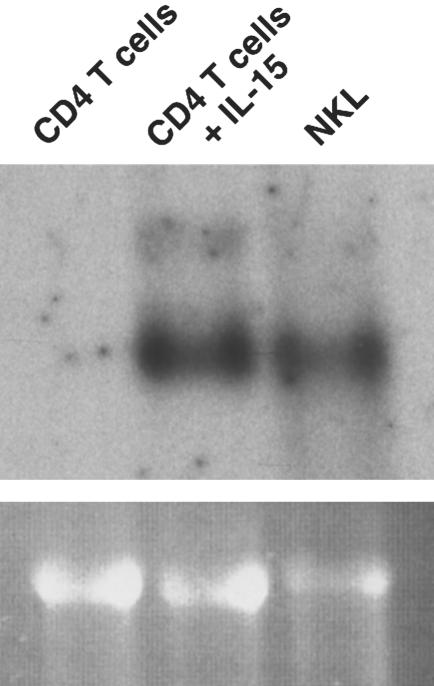
Fig. 3.
Cytokine-induced expression of NKG2D mRNA. Exposure of CD4+ T cells sorted from normal PBL for 6 days to IL-15 results in de novo induction of NKG2D mRNA. The NKL NK cell line serves as a positive control. This result confirms that the absence of NKG2D on uninduced CD4 T cells is not caused by a lack of its DAP10 partner protein, which is required for surface expression.
As with IL-15, TNF-α is a key cytokine in the immunopathology of RA and induced NKG2D expression on CD4+NKG2D– T cells among control and RA PBL (Fig. 2B; data not shown). The presence of both cytokines was confirmed in all of 10 RA peripheral blood serum samples, at concentrations of 6.4–13.3 pg/ml (mean 8.2 pg/ml) and 16.5–52.2 pg/ml (mean 24.4 pg/ml), respectively (data not shown). Exposure to IL-10, but not to IL-12 and IFN-γ, resulted in less pronounced and variable induction of NKG2D. T cell antigen receptor complex stimulation with anti-CD3 transiently induced NKG2D on some CD4 T cells (data not shown).
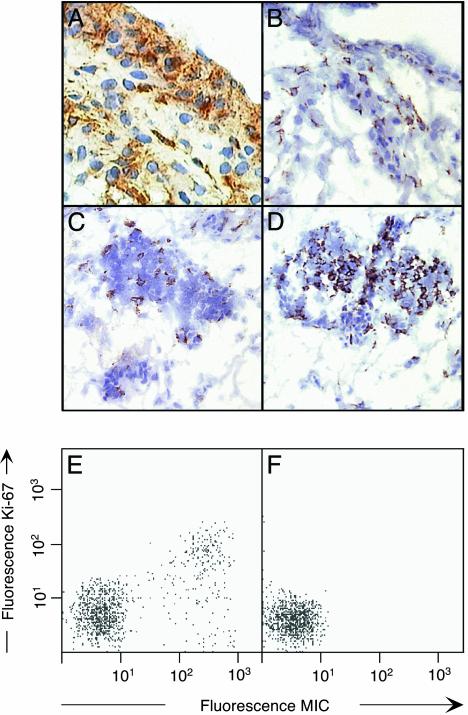
Aberrant Expression of MIC in RA Synovium. To explore the significance of CD4 T cell expression of NKG2D in the immunopathology of RA, frozen sections of disease synovial tissue specimens were tested for the presence of MIC by immunohistochemistry using mAb 6D4, which is specific for MICA and MICB, and isotype-matched negative control antibody (10). As observed by peroxidase substrate staining, all tissue specimens contained numerous positive cells. MIC+ synoviocytes of spindle-shaped fibroblast-like and more rounded morphologies were distributed throughout the synovial lining and sublining areas and were often located close to, or interspersed with, lymphocytic aggregates (Fig. 4 A and C). They were in close contact with NKG2D+ lymphocytes, presumably CD4+ T cells that were present throughout the synovial lining and in organized lymphoid microstructures (refs. 18 and 29 and Fig. 4 B and D). Rheumatoid synovial hyperplasia consists of fibroblasts and activated macrophages. The former have features of immortalized transformed cells and proliferate aggressively (30), which may explain the induced expression of MIC (10). This was confirmed by two-color staining of permeabilized synovial cell suspensions with antibodies against the nuclear Ki-67 proliferation marker and MIC (31). Analysis by flow cytometry revealed that the presence of MIC was strongly but not completely associated with expression of Ki-67 (Fig. 4E). Control staining of cell suspensions derived from osteoarthritis tissue specimens gave negative results (Fig. 4F). Thus, in accord with previous evidence obtained with fibroblast and epithelial cell lines, expression of MIC was induced in proliferating rheumatoid synoviocytes (10).
Fig. 4.
Expression of MIC and NKG2D on proliferating RA synoviocytes and lymphocytes, respectively. Micrographs of frozen sections from RA synovium stained for MIC (A and C) and NKG2D (B and D) by brown diaminobenzidine peroxidase substrate. Nuclei are counterstained with hematoxylin. A and B display parts of the synovial lining layer and underlying sublining areas. C and D show lymphocytic aggregates within sublining areas. (E) Two-color immunofluorescence staining of cell suspensions from RA synovium with anti-MIC and, after permeabilization, with anti Ki-67. (F) Identically treated cell suspensions from osteoarthritis synovium showed no MIC expression.
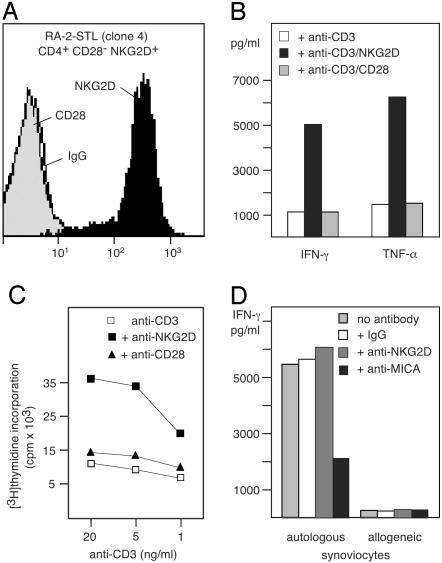
NKG2D Stimulates CD4+CD28– T Cell Autoreactivity. CD4+CD28– T cells resemble NKT cells as they secrete large amounts of IFN-γ and express perforin and granzyme B, which confer cytotoxic capacity (21, 32). To test the function of NKG2D, we established each five CD4+CD28–NKG2D+ T cell clones from one RA synovial tissue specimen and two RA PBL samples (Fig. 5A). These clones represented a small minority of ≈2%, as most of the sorted T cells grown in culture lost NKG2D in the absence of IL-15. In antibody-dependent cytotoxicity assays, ligation of NKG2D did not induce redirected lysis of FcγR+ mouse mastocytoma P815 cells by any of the 15 T cell clones, although anti-CD3 was effective, thus confirming their cytotoxic capacity (data not shown). Consistent with previous results obtained with antigen-specific CD8 αβ T cells, no cytotoxicity was scored against the C1R-MICA transfectant B-cell line (ref. 13 and data not shown). However, mAb crosslinking of NKG2D strongly augmented anti-CD3-triggered release of IFN-γ and TNF-α by all T cell clones and stimulated T cell proliferation (Fig. 5 B and C). Crosslinking of NKG2D alone had no effect (data not shown). Thus, as with antigen-specific effector CD8 αβ T cells, NKG2D costimulated RA CD4+CD28– T cells (13). These results imply that NKG2D may contribute to the frequent expansion of these T cells in RA.
Fig. 5.
NKG2D-mediated costimulation and autoreactivity of CD4+CD28–NKG2D+ T cells. (A) Immunofluorescence staining of the RA-2 synovial T lymphocyte (STL) clone 4. (B) Secretion of IFN-γ and TNF-α by RA-2-STL after stimulation with solid-phase anti-CD3 and anti-NKG2D mAbs. (C) Stimulation of T cell proliferation by anti-CD3 together with anti-NKG2D mAbs. (D) Secretion of IFN-γ upon stimulation with irradiated autologous but not allogeneic synoviocytes. T cell to stimulator cell ratios were 1:1.
CD4+CD28– T cells are thought to promote the formation and maintenance of RA inflammatory lesions mainly through IFN-γ release. IFN-γ perpetuates synoviocyte pathology, which is associated with secretion of TNF-α, IL-15, and tissue-injurious metalloproteinases by synovial fibroblasts and macrophages (33). We tested whether ligation of NKG2D by MIC+ RA synoviocytes could induce cytokine production by synovial CD4+CD28–NKG2D+ T cell clones. T cells were stimulated with autologous or mismatched RA synoviocytes, and release of IFN-γ and TNF-α was measured in the presence or absence of anti-MIC or anti-NKG2D mAbs. Cytokine release was stimulated by the autologous but not the allogeneic synoviocytes and was abrogated by anti-MIC mAb. Anti-NKG2D moderately superinduced cytokine production as previously found with antigen-specific CD8 T cell clones (ref. 13 and Fig. 5D).
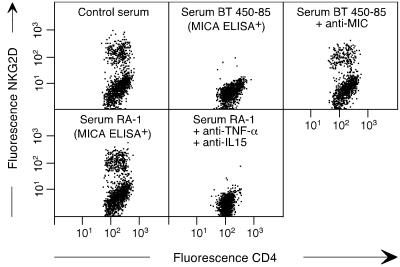
TNF-α and IL-15 Counteract Down-Modulation of NKG2D by Soluble MIC in RA Patient Serum. Binding of MIC induces down-modulation of NKG2D, which may normally serve to prevent chronic T cell stimulation and limit autoreactive bystander T cell activation. Many epithelial tumors cause a systemic down-modulation of NKG2D by shedding of soluble MIC, which is presumably mediated by metalloproteinases (17, 34). Because metalloproteinases are secreted by RA synoviocytes, we tested peripheral blood serum samples from RA patients for the presence of soluble MICA by using an ELISA (17). Positive results were obtained with all of the ten samples tested, which contained 2.7–30.6 ng/ml (mean 5.8 ng/ml) of soluble MICA (data not shown). This raised the question of why NKG2D was expressed at high levels on RA CD4+CD28– T cells as well as on CD8+ T cells. As expected, incubation of RA patient PBMC with soluble MIC containing serum from a breast cancer patient diminished NKG2D expression on CD4+NKG2D+ T cells. This effect was abrogated in the presence of the anti-MIC mAb 6D4 (Fig. 6) (17). By contrast, RA patient serum failed to down-modulate NKG2D because of the presence of TNF-α and IL-15 (Fig. 6). Thus, the ligand-induced down-modulation of NKG2D in RA patients was compensated by the opposite effect of its cytokine-mediated induction.
Fig. 6.
Failure of soluble MIC in RA patient serum to down-modulate NKG2D due to the presence of TNF-α and IL-15. (Upper) Two-color staining of gated CD3+CD4+ T cells among RA PBL incubated with control serum or serum from a breast tumor patient containing soluble MIC (serum BT 450–85; ref. 17) in the absence or presence of anti-MIC mAb. (Lower) Incubation of RA PBL with autologous RA patient serum containing soluble MIC in the absence or presence of anti-TNF-α and anti-IL-15 mAbs. See the text for further explanations.
Conclusions. The self-perpetuating pathology in RA is caused by the interplay between lymphocytic infiltrates, synovial macrophages and fibroblasts, and their respective products. Destruction of cartilage results from pannus invasion, which is composed of activated macrophages and proliferating fibroblasts. Both cell types secrete metalloproteinases and other enzymes that degrade surrounding cartilage and extracellular matrix and produce inflammatory cytokines such as TNF-α and IL-15 (18, 28, 30, 33). These activities are promoted by CD4+CD28– T cell-derived IFN-γ and TNF-α, as indicated by T cell depletion and cytokine reconstitution experiments (33). Some RA peripheral blood-derived CD4+CD28– T cell clones express the activating KIR2DS2 isoform unopposed by inhibitory receptors, which may be responsible for breaches of tolerance (6, 7, 35). However, the frequency of these T cells in RA is unknown because activating or inhibitory KIR isoforms cannot be readily distinguished with antibody reagents.
Our results show that substantial numbers of RA synovial and circulating CD4 T cells, mostly those lacking CD28, express NKG2D, most likely as a result of their exposure to TNF-α and IL-15. Moreover, its MIC ligands are induced on proliferating RA synoviocytes and may thus contribute to joint disease perpetuation and progression by costimulation of CD4+CD28– T cell cytokine production and proliferation. The additional occurrence of CD4+CD28– T cells in Wegener's granulomatosis, Sjögren's syndrome, and insulin-dependent diabetes mellitus suggests a potentially broader role of NKG2D and its ligands in autoimmune diseases (20, 23, 36). As with many epithelial tumor patients, RA synoviocytes shed substantial amounts of soluble MIC into the circulation, which may be a useful prognostic indicator. However, soluble MIC fails to down-modulate NKG2D because of the counteractive function of TNF-α and IL-15. Thus, unmasking NKG2D modulation by soluble MIC in RA patients may represent one among various therapeutic effects of antiinflammatory agents targeting TNF-α or IL-15.
Acknowledgments
We thank Heather Thomas, Guoping Ma, and Keng Wong for technical assistance. This work was supported by National Institutes of Health Grants AI30581 and AI52319.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RA, rheumatoid arthritis; MICA and MICB, major histocompatibility complex class I-related chains A and B; KIR, killer cell inhibitory receptor; TNF-α, tumor necrosis factor α; NK, natural killer; PBMC, peripheral blood mononuclear cells; PBL, peripheral blood lymphocytes.
References
- 1.Ravetch, J. V. & Lanier, L. L. (2000) Science 290, 84–89. [DOI] [PubMed] [Google Scholar]
- 2.Lanier, L. L. (2001) Nat. Immunol. 2, 23–27. [DOI] [PubMed] [Google Scholar]
- 3.Mingari, M. C., Schiavetti, F., Ponte, M., Vitale, C., Maggi, E., Romagnani, S., Demarest, J., Pantaleo, G., Fauci, A. S. & Moretta, L. (1996) Proc. Natl. Acad. Sci. USA 93, 12433–12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speiser, D. E., Valmori, D., Rimoldi, D., Pittet, M. J., Lienard, D., Cerundolo, V., MacDonald, H. R., Cerottini, J.-C. & Romero, P. (1999) Eur. J. Immunol. 29, 1990–1999. [DOI] [PubMed] [Google Scholar]
- 5.Moser, J. M., Gibbs, J., Jensen, P. E. & Lukacher, A. E. (2002) Nat. Immunol. 3, 189–195. [DOI] [PubMed] [Google Scholar]
- 6.Namekawa, T. M., Snyder, M. R., Yen, J.-H., Goehring, B. E., Leibson, P. J., Weyand, C. M. & Goronzy, J. J. (2000) J. Immunol. 165, 1138–1145. [DOI] [PubMed] [Google Scholar]
- 7.Yen, J.-H., Moore, B. E., Nakajima, T., Scholl, D., Schaid, D. J., Weyand, C. M. & Goronzy, J. J. (2001) J. Exp. Med. 193, 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer, S., Groh, V., Wu, J., Steinle, A., Phillips, J. H., Lanier, L. L. & Spies, T. (1999) Science 285, 727–729. [DOI] [PubMed] [Google Scholar]
- 9.Groh, V., Bahram, S., Bauer, S., Herman, A., Beauchamp, M. & Spies, T. (1996) Proc. Natl. Acad. Sci. USA 93, 12445–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groh, V., Steinle, A., Bauer, S. & Spies, T. (1998) Science 279, 1737–1740. [DOI] [PubMed] [Google Scholar]
- 11.Groh, V., Rhinehart, R., Secrist, H., Bauer, S., Grabstein, K. H. & Spies, T. (1999) Proc. Natl. Acad. Sci. USA 96, 6879–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das, H., Groh, V., Kuijl, C., Sugita, M., Morita, C. T., Spies, T. & Bukowski, J. F. (2001) Immunity 15, 83–93. [DOI] [PubMed] [Google Scholar]
- 13.Groh, V., Rhinehart, R., Randolph-Habecker, J., Topp, M. S., Riddell, S. R. & Spies, T. (2001) Nat. Immunol. 2, 255–260. [DOI] [PubMed] [Google Scholar]
- 14.Tieng, V., Le Bouguenec, C., du Merle, L., Bertheau, P., Desreumaux, P., Janin, A., Charron, D. & Toubert, A. (2002) Proc. Natl. Acad. Sci. USA 99, 2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu, J., Song, Y., Bakker, A. B. H., Bauer, S., Spies, T., Lanier, L. L. & Phillips, J. H. (1999) Science 285, 730–732. [DOI] [PubMed] [Google Scholar]
- 16.Roberts, A. I., Lee, L., Schwartz, E., Groh, V., Spies, T., Ebert, E. C. & Jabri, B. (2001) J. Immunol. 167, 5527–5530. [DOI] [PubMed] [Google Scholar]
- 17.Groh, V., Wu, J., Yee, C. & Spies, T. (2002) Nature 419, 734–738. [DOI] [PubMed] [Google Scholar]
- 18.Feldmann, M., Brennan, F. M. & Maini, R. N. (1996) Annu. Rev. Immunol. 14, 397–440. [DOI] [PubMed] [Google Scholar]
- 19.Ivashkiv, L. B. (1996) Adv. Immunol. 63, 337–376. [DOI] [PubMed] [Google Scholar]
- 20.Miller, G., Nepom, G. T., Reich, M. B. & Thomas, J. W. (1993) Hum. Immunol. 36, 219–226. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt, D., Goronzy, J. J. & Weyand, C. M. (1996) J. Clin. Invest. 97, 2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martens, P. B., Goronzy, J. J., Schaid, D. & Weyand, C. M. (1997) Arthritis Rheum. 40, 1106–1114. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto, T., Katayama, I. & Nishioka, K. (1998) Eur. J. Dermatol. 8, 248–251. [PubMed] [Google Scholar]
- 24.Wagner, U., Pierer, M., Kaltenhauser, S., Wilke, B., Seidel, W., Arnold, S. & Hantzschel, H. (2003) Eur. J. Immunol. 33, 79–84. [DOI] [PubMed] [Google Scholar]
- 25.Snyder, M. R., Muegge, L.-O., Offord, C., O'Fallon, W. M., Bajzer, Z., Weyand, C. M. & Goronzy, J. J. (2002) J. Immunol. 168, 3839–3846. [DOI] [PubMed] [Google Scholar]
- 26.McInnes, I. B., Al-Mughales, J., Field, M., Leung, B. P., Huang, F.-P., Dixon, R., Sturrock, R. D., Wilkinson, P. C. & Liew, F. Y. (1996) Nat. Med. 2, 175–182. [DOI] [PubMed] [Google Scholar]
- 27.McInnes, I. B., Leung, B. P., Sturrock, R. D., Field, M. & Liew, F. Y. (1997) Nat. Med. 3, 189–195. [DOI] [PubMed] [Google Scholar]
- 28.Kurowska, M., Rudnicka, W., Kontny, E., Janicka, I., Chorazy, M., Kowalczewski, J., Ziolkowska, M., Ferrari-Lacraz, S., Strom, T. B. & Maslinski, W. (2002) J. Immunol. 169, 1760–1767. [DOI] [PubMed] [Google Scholar]
- 29.Young, C. L., Adamson, T. C., Vaughan, J. H. & Fox, R. I. (1984) Arthritis Rheum. 27, 32–39. [DOI] [PubMed] [Google Scholar]
- 30.Krause, A., Scaletta, N., Ji, J.-D. & Ivashkiv, L. B. (2002) J. Immunol. 169, 6610–6616. [DOI] [PubMed] [Google Scholar]
- 31.Scholzen, T. & Gerdes, J. (2000) J. Cell. Physiol. 182, 311–322. [DOI] [PubMed] [Google Scholar]
- 32.Namekawa, T., Wagner, U. G., Goronzy, J. J. & Weyand, C. M. (1998) Arthritis Rheum. 41, 2108–2116. [DOI] [PubMed] [Google Scholar]
- 33.Klimiuk, P. A., Yang, H., Goronzy, J. J. & Weyand, C. M. (1999) Clin. Immunol. 90, 65–78. [DOI] [PubMed] [Google Scholar]
- 34.Salih, H. R., Rammensee, H.-G. & Steinle, A. (2002) J. Immunol. 169, 4098–4102. [DOI] [PubMed] [Google Scholar]
- 35.Snyder, M. R., Lucas, M., Vivier, E., Weyand, C. M. & Goronzy, J. J. (2003) J. Exp. Med. 197, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komocsi, A., Lamprecht, P., Csernok, E., Muller, A., Holl-Ulrich, K., Seitzer, U., Moosig, F., Schnabel, A. & Gross, W. L. (2002) Am. J. Pathol. 160, 1717–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]