Abstract
Protein kinase C (PKC) and Syk protein tyrosine kinase play critical roles in immune cell activation including that through the high-affinity IgE receptor, FcεRI. Mechanisms by which PKC activation leads to the activation of Ras, a family of GTPases essential for immune cell activation, have been elusive. We present evidence that Tyr-662 and Tyr-658 of PKCβI and PKCα, respectively, are phosphorylated by Syk in the membrane compartment of FcεRI-stimulated mast cells. These phosphorylations require prior PKC autophosphorylation of the adjacent serine residues (Ser-661 and Ser-657, respectively) and generate a binding site for the SH2 domain of the adaptor protein Grb-2. By recruiting the Grb-2/Sos complex to the plasma membrane, these conventional PKC isoforms contribute to the full activation of the Ras/extracellular signal-regulated kinase signaling pathway in FcεRI-stimulated mast cells.
Engagement of multichain immune recognition receptors, including antigen receptors and the high-affinity IgE receptor, FcεRI, induces the activation of a number of protein kinases, among which protein tyrosine kinase (PTK) Syk and the protein kinase C (PKC) family of serine/threonine kinases play crucial roles in immune cell activation (1–4). Receptor crosslinking elicits the enzymatic activation of receptor-bound Src family PTKs such as Lyn. These kinases phosphorylate tyrosine residues in the immunoreceptor tyrosine-based activation motifs (ITA Ms) in signaling subunits of receptor. Tyrosine-phosphorylated ITAMs recruit Src family and Syk kinases through Src homology 2 (SH2) domain–phosphotyrosine interactions and activate these kinases. Syk in concert with Bruton's tyrosine kinase (Btk), another PTK that is critical for B and mast cell activation, phosphorylates and activates phospholipase C (PLC)-γ. PLC-γ hydrolyzes phosphatidylinositol 4,5-bisphosphate into diacylglycerol and inositol 1,4,5-trisphosphate. Diacylglycerol activates several PKC isoforms and 1,4,5-trisphosphate recruits Ca2+ from intracellular storage sites.
The PKC family of serine/threonine kinases play crucial roles in a plethora of biological functions such as proliferation, differentiation, development, and more specialized cellular functions (4–6). Based on cofactor requirements and structure, PKC family members are divided into the Ca2+/diacylglycerol-regulated conventional isoforms (α, βI, βII, and γ), the Ca2+-independent, but diacylglycerol-regulated isoforms (δ, ε, η, and θ), and the Ca2+/diacylglycerol-independent atypical isoforms (ζ and λ/ι). Recently, PKCβI was shown to be regulated by Syk and Btk, apparently through the activation of PLC-γ, and to be required for the regulation of cytokine gene expression in FcεRI-stimulated mast cells (7). Activation of the Ras/extracellular signal-regulated kinase (ERK) pathway is another critical event for immune cell activation, leading to transcriptional regulation of cytokine genes, translational regulation, and other effector functions (8, 9). In mast cells, Ras activation leads to activation of cytosolic phospholipase A2, thus release of arachidonic acid (10, 11). Ras activity is cycled between an inactive GDP-bound state and an active GTP-bound state. The ratio of GTP-bound Ras to GDP-bound Ras is negatively regulated by GTPase-activating proteins (GAPs) and positively regulated by guanine nucleotide exchange factors (GEFs) (12). GTP-bound Ras activates the canonical cascade of three kinases, i.e., c-Raf-1 → mitogen-activated protein kinase/ERK kinase (MEK) → ERK. Although several mechanisms including both PKC-dependent and -independent routes have been proposed to explain how this pathway is activated in immune cells (13–17), the exact mechanism by which PKC regulates the Ras/ERK pathway has been an enigma for a long time. In this article, we describe a mechanism for Ras activation that depends on PKCα or PKCβI, as well as Syk.
Materials and Methods
Cell Culture and Stimulation. Bone marrow cells derived from wild-type, btk-/- (18), and PKCβ-/- (19) mice were cultured in IL-3-containing medium for 4–6 weeks to generate >95% pure populations of mast cells (20). Cells were sensitized overnight with anti-dinitrophenyl (DNP) IgE mAb and stimulated with the antigen, DNP-human serum albumin conjugates (a kind gift from Teruko Ishizaka, La Jolla Institute for Allergy and Immunology, San Diego). Retroviral transfection of PKCβ-/- mast cells was done as described (21). Wild-type, Syk-deficient variant, and syk cDNA-transfected syk- RBL-2H3 cells were described (22).
Immunoblotting Analysis and Antibodies. Subcellular fractionation was performed as described (23). Cells and subcellular fractions were solubilized in 1% Nonidet P-40-containing lysis buffer (20 mM Tris·HCl, pH 8.0/0.15 M NaCl/1 mM EDTA/1 mM sodium orthovanadate/1 mM phenylmethylsulfonyl fluoride/10 μg/ml aprotinin/10 μg/ml leupeptin/25 μM p-nitrophenyl p′-guanidinobenzoate/1 μM pepstatin/0.1% sodium azide). Proteins in cleared cell lysates or subcellular fractions were either immunoprecipitated before, or directly analyzed by, SDS/PAGE, followed by electroblotting onto poly(vinylidene difluoride) membranes (Millipore). Antibodies used for immunoprecipitation and blotting were: anti-PKCα (C-20), anti-PKCβI (C-16), anti-PKCβII (C-18), anti-Lyn (44), anti-Btk (M138), anti-Syk (C-20), anti-GST, and anti-Grb-2, all from Santa Cruz Biotechnology; anti-phospho-PKC (pan), a gift from M. Comb, Cell Signaling Technology, Beverly, MA; anti-Sos, anti-Ras, and anti-phosphotyrosine 4G10 mAb from Upstate Biotechnology; anti-phospho-ERK from Cell Signaling Technology; and anti-ERK from Zymed (South San Francisco, CA). Proteins reactive with primary antibody were visualized with an horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence reagents (Perkin–Elmer Life Sciences, Boston).
In Vitro Kinase Assays. Active Btk, Lyn, and Syk molecules were immunoprecipitated from pervanadate-stimulated MCP-5 murine mast cells with respective antibodies (all from Santa Cruz Biotechnology). Immune complexes were incubated with 5 μgof synthetic peptides in the kinase buffer (50 mM Hepes, pH 7.4/10 mM MgCl2/10 mM MnCl2/0.1 μM ATP) in the presence of 10 μCi [γ-32P]ATP (1 Ci = 37 GBq). Reaction products were analyzed by SDS/PAGE and autoradiography of dried gels.
PKC Kinase Assay. COS-7 cells were electroporated with plasmid constructs. Transfected cells were lysed for in vitro PKC kinase assays and used for immunoprecipitation with anti-hemagglutinin (12CA5, Roche Molecular Biochemicals) before SDS/PAGE and immunoblotting with anti-hemagglutinin or anti-phospho-PKC (pan), which was used to detect Thr-641-phosphorylated PKCβI.
Ras Assay. Cell lysates were incubated with GST-Raf-1 RBD agarose beads (Upstate Biotechnology). GTP-bound Ras precipitated with the beads were detected by SDS/PAGE and immunoblotting with anti-pan-isoform-specific Ras antibody (Upstate Biotechnology).
Results
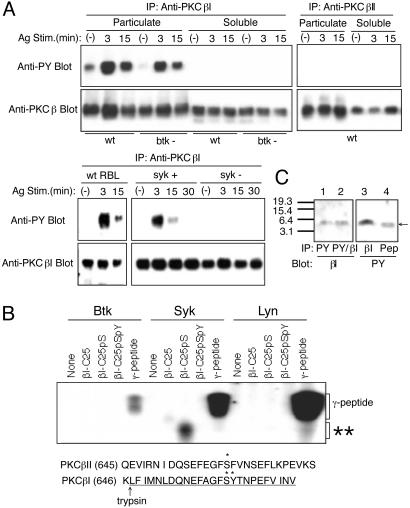
Syk Phosphorylates Tyr-662 in PKCβI on FcεRI Stimulation. Given the importance of PKCβI in FcεRI signal transduction (7, 24–26), we further characterized the role of this kinase in mast cell signal transduction. We found that PKCβI was tyrosine-phosphorylated in mouse bone marrow-derived cultured mast cells (BMMCs) on FcεRI crosslinking, whereas PKCβII was not (Fig. 1A). Tyrosine phosphorylation of PKCβI occurred nearly exclusively in the membrane compartment (Fig. 1 A). We next explored which PTK is required for this tyrosine phosphorylation. In contrast to wild-type RBL-2H3 rat mast cells, tyrosine phosphorylation of PKCβI was nearly undetectable in Syk-deficient RBL-2H3 cells (ref. 22; Fig. 1 A). Transfection of Syk-deficient RBL-2H3 cells with syk cDNA reconstituted tyrosine phosphorylation of PKCβI. In contrast, PKCβI was tyrosine-phosphorylated in FcεRI-stimulated BMMCs derived from btk-/- mice (18), albeit to a lesser extent than in wild-type cells. Therefore, we conclude that Syk is required for tyrosine phosphorylation of PKCβI.
Fig. 1.
Tyr-662 of PKCβI is phosphorylated by Syk in vitro and in vivo. (A) IgE-sensitized wild-type (wt) and btk-/- BMMCs were stimulated with antigen and were fractionated into particulate and cytosolic compartments. Proteins in the particulate fraction equivalent to 2 × 107 cells and cytosolic proteins equivalent to 106 cells were used for immunoprecipitation, followed by immunoblotting with 4G10 anti-phosphotyrosine mAb (anti-PY). The same blots were reprobed with the precipitating antibodies. Wild-type, Syk-deficient (syk-), or syk-reconstituted syk- (syk+) RBL-2H3 cells were similarly analyzed. (B) βI-C25 peptides were incubated with Btk, Syk, or Lyn, which was immunopurified from pervanadate-stimulated MCP-5 mast cells, in the presence of [γ-32P]ATP. Reactions were analyzed by SDS/PAGE and autoradiography. βI-C25 is a nonphosphorylated form, βI-C25pS is a peptide phosphorylated at position S661, and βI-C25pSpY is a peptide phosphorylated at positions S661 and Y662. As a control, a peptide corresponding to the C-terminal 37-aa residues of mouse FcεRI γ subunit was used. **, the position of the phosphorylated βI-C25pS. The C-terminal sequences of PKCβI and PKCβII are shown in a single-letter code (Lower). *, the in vivo phosphorylation sites of S660 of PKCβII and S661 and Y662 of PKCβI. The βI-C25 peptide sequence is underlined, and the trypsin cleavage site N-terminal to the βI-C25 peptide is also shown. (C) IgE-sensitized MCP-5 cells were stimulated by antigen for 10 min. PKCβI was isolated and digested with TPCK trypsin. Digests were immunoprecipitated with anti-PKCβI (lane 3), 4G10 (lane 1), or both (lane 2). Immunoprecipitated peptides were separated by a 10–20% tricine gel and detected by immunoblotting with anti-PKCβI (C-16) or 4G10. As a position marker, βI-C25pSpY peptide was run in the same gel (lane 4).
PKCβI and PKCβII are generated by alternative splicing of a common precursor mRNA, and differ only at their carboxyl termini (27). Based on this difference, we hypothesized that PKCβI was phosphorylated on a tyrosine residue (Y662) in its unique 50-residue C-terminal region. There is no tyrosine residue in the corresponding 52-residue region of PKCβII. To test this hypothesis, a synthetic peptide, βI-C25, comprising the C-terminal 25-residue sequence of mouse PKCβI and phosphorylated versions of this peptide were submitted to kinase assays with Syk, Btk, or Lyn. Neither βI-C25 nor a similar PKCβII peptide (βII-C28) was phosphorylated by these kinases (Fig. 1B and data not shown). Because the serine residue (S660) of PKCβII (corresponding to S661 of PKCβI) is an in vivo autophosphorylation site (28, 29), we next tested a peptide, βI-C25pS, in which the serine residue corresponding to S661 in βI is phosphorylated. Remarkably, the βI-C25pS peptide was phosphorylated by Syk, but not by Btk or Lyn (Fig. 1B). As expected, the βI-C25pSpY peptide phosphorylated on both S661 and Y662 residues was not phosphorylated by any of these kinases. These findings support the conclusion that the phosphoserine (S661) mimics an acidic residue in front of the phosphoacceptor tyrosine (Y662) similar to that in sequences of the substrates favored by Syk (30). Consistent with the findings, recombinant PKCβI was also tyrosine-phosphorylated by recombinant Syk in vitro (data not shown).
We next examined whether PKCβI is phosphorylated at Y662 in FcεRI-stimulated mast cells. Because it is easy to culture MCP-5 mouse mast cells in a large scale, and FcεRI stimulation induced tyrosine phosphorylation of PKCβI, but not PKCβII, in MCP-5 cells, we used this cell line for phosphopeptide mapping. PKCβI was immunoprecipitated from FcεRI-stimulated MCP-5 mouse mast cells and identified by immunoblotting. PKCβI bands on the blot were excised and subjected to extensive digestion with trypsin. Trypsin digests were immunoprecipitated with anti-PKCβI or anti-phosphotyrosine antibodies, followed by immunoblotting. Comparison of the tyrosine-phosphorylated band with the band detected by anti-PKCβI antibody specific for the C-terminal 16 residues allowed us to identify Y662 as a tyrosine-phosphorylated residue (Fig. 1C). In a control experiment, anti-PKCβII antibody specific for the C-terminal 18 residues did not immunoprecipitate a tryptic peptide corresponding to βI-C25pSpY, which could be blotted with antiphosphotyrosine mAb (data not shown). Therefore, these results demonstrate that PKCβI is phosphorylated on Y662 by Syk in vitro and in vivo. The reduced tyrosine phosphorylation of PKCβI (≈40% lower) in btk -/- mast cells (Fig. 1 A) is likely because of its reduced translocation to the membrane (7), where it is phosphorylated by Syk.
Conventional PKC isoforms are phosphorylated by PDK1 on the activation loop (T500 in PKCβII), and by subsequent autophosphorylation at two C-terminal sites: the turn motif (T641 in PKCβII) and hydrophobic motif (S660 in PKCβII). Phosphorylation at these positions process PKC into a translocation-competent species that partitions in the cytosol (31). Mutational analysis (see Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site, www.pnas.org) indicated that phosphorylation at Y662 before S661 phosphorylation is inhibitory for the maturation of the enzyme. The results suggest that PKCβI initially phosphorylated at T500, T642, and S661 is recruited to the plasma membrane, where Y662 is subsequently phosphorylated by Syk.
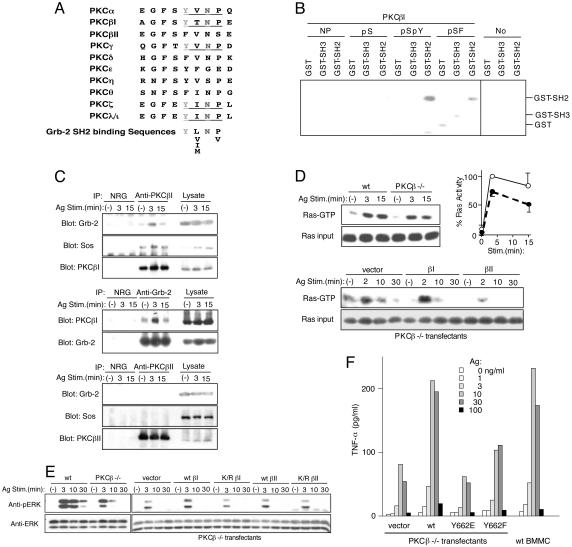
Phosphorylation of Tyr-662 in PKCβI Creates the Binding Site for Grb-2. Notably, phosphorylated Y662, followed by three residues (pTyr-Thr-Asn-Pro), generates a potential binding site for the SH2 domain of the adaptor protein Grb-2 (ref. 32 and Fig. 2A). Grb-2, complexed with the Ras GEF protein Sos, was shown to recruit Ras to the plasma membrane and activate it in response to a number of stimuli including peptide growth factors (33, 34). We therefore tested the model that phosphorylation of PKCβI-Y662 could lead to Grb-2 recruitment and downstream activation of Ras. We initially examined whether the N-terminally biotinylated βI-C25pSpY peptide immobilized onto avidin-agarose could interact with the SH2 domain of Grb-2. Strikingly, βI-C25pSpY precipitated a Grb-2 SH2-GST fusion protein, but not Grb-2-NSH3-GST or GST alone (Fig. 2B). In contrast, βI-C25 and βI-C25pS peptides interacted minimally with Grb-2 SH2-GST. We also tested whether the βI-C25pSpY peptide interacts with Grb-2 in MCP-5 cell lysates. Immunoblotting of precipitated proteins clearly demonstrated interaction of the phosphopeptide with endogenous Grb-2 (data not shown). In contrast, βI-C25pS failed to precipitate Grb-2 from the cell lysates.
Fig. 2.
Tyr-662 phosphorylated PKCβI interacts with Grb-2/Sos complexes and regulates the Ras/ERK pathway. (A) Alignment of hydrophobic motif sequences of various PKC isoforms. Consensus Grb-2 SH2-binding sequences (32) are included. (B) Direct interaction between C-terminal peptides of PKCβI and the SH2 domain of Grb2. Immobilized N-terminally biotinylated βI-C25 peptides were incubated with GST, GST-NSH3 (N-terminal SH3 domain), or GST-SH2. Bound GST fusion proteins were analyzed by SDS/PAGE and by immunoblotting with anti-GST. Avidin agarose beads without peptide conjugation (No) served as a negative control. Positions of GST, GST-NSH3, and GST-SH2 are indicated. (C) MCP-5 cells were stimulated with IgE and antigen. Immunoprecipitates were analyzed by immunoblotting with the indicated antibodies. (D) BMMCs from wild-type and PKCβ-/- mice or PKCβ-/- BMMCs transfected with the indicated vectors were stimulated as above. Cell lysates were incubated with GST-Raf-1 RBD agarose beads. GTP-bound Ras precipitated with the beads were detected by immunoblotting with anti-Ras antibody. Relative Ras-GTP amounts (normalized against that in wild-type cells stimulated for 3 min) in three experiments were plotted (Right Upper). ○ and •, the values in wild-type and PKCβ-/- cells, respectively. (E) Lysates prepared as in D were analyzed by immunoblotting with anti-phospho-ERK antibody. The same blots were reprobed with anti-ERK antibody (Zymed). (F) PKCβ-/- BMMC transfectants were stimulated with IgE and antigen for 3 h. TNF-α secreted into culture media was measured by ELISA. Also included are amounts of TNF-α secreted from similarly stimulated wild-type BMMCs that had been cultured in stem cell factor.
We next examined whether PKCβI interacts with Grb-2 in vivo. Lysates from unstimulated or IgE- and antigen-stimulated MCP-5 mast cells were immunoprecipitated with anti-PKCβI antibody, and analyzed by blotting with anti-Grb-2. As shown in Fig. 2C, there was a low level of Grb-2 protein in anti-PKCβI immune complexes in unstimulated cells, and much more significant increase in Grb-2 protein was detected after 3-min stimulation. Coimmunoprecipitation levels returned to the basal levels ≈30 min after FcεRI crosslinking (Fig. 2C and data not shown). These kinetics of coimmunoprecipitation were similar to those of PKCβI tyrosine phosphorylation. Conversely, PKCβI was also detected in immune complexes precipitated with anti-Grb-2 antibody with similar kinetics. In a control experiment, Grb-2 was not detected in anti-PKCβII immunoprecipitates (Fig. 2C). Together, these results demonstrate an activation-dependent, SH2-phosphotyrosine-mediated interaction between PKCβI and Grb-2.
PKCβI–Grb-2 Interaction Appears to Contribute to the Activation of Ras and ERK. Because tyrosine phosphorylation of PKCβI occurs only in the membrane compartment, it suggests that the PKCβI–Grb-2 interaction might contribute to the activation of Ras, a plasma membrane protein. To further investigate this possibility, we next examined whether PKCβI-interacting Grb-2 is also associated with Sos. As shown in Fig. 2C, Sos was detected in immune complexes precipitated with anti-PKCβI in resting cells and amounts of coprecipitated Sos were increased on FcεRI stimulation. We next compared Ras activity between wild-type and PKCβ-/- BMMCs (Fig. 2D). GTP-bound active Ras, low at the basal level, increased on FcεRI stimulation in cells from both strains. However, the peak activation level in PKCβ-/- BMMCs was ≈25% lower, compared with wild-type cells. In keeping with these results, ERK activation was also 30–50% lower in PKCβ-/- cells (Fig. 2E). Reconstitution of PKCβI expression in these cells by retroviral transfection increased Ras/ERK activation by 30–50%, indicating full restoration of Ras/ERK activation (Fig. 2 D and E). However, PKCβI expression levels in transfectants were several-fold higher than that of the endogenous PKCβI in wild-type BMMCs (data not shown). Of note is that the transfectants that have been cultured in the presence of stem cell factor, exhibit more transient Ras/ERK activation on FcεRI stimulation than nontransfected BMMCs. In contrast, reconstitution of PKCβII expression did not enhance ERK activation. Furthermore, reconstitution with a nonphosphorylatable mutant (Y662F) of PKCβI did not restore ERK activation (data not shown), which was consistent with the reduced affinity of the βI-C25pSF peptide for the Grb-2 SH2 domain (Fig. 2B). Finally, we measured tumor necrosis factor α (TNF-α) secreted into media from FcεRI-stimulated wild-type and PKCβ-/- BMMCs (Fig. 2F). PKCβ-/- cells secreted significantly less TNF-α than did wild-type cells. Reconstitution with the wild-type PKCβI, but not PKCβI-Y662F, increased TNF-α secretion. These results are consistent with TNF-α regulation by ERK through the nucleocytoplasmic transport of TNF-α mRNA (35). Together, these data indicate that the PKCβI–Grb-2 interaction contributes to the full activation of the Ras/ERK pathway.
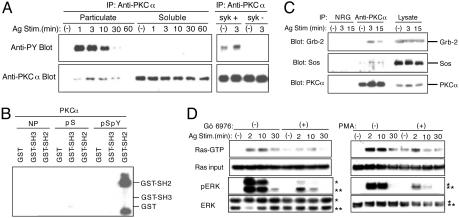
Phosphorylation of Tyr-658 in PKCα Also Appears to Contribute to the Ras/ERK Activation. Comparison of the C-terminal region among PKC isoforms indicates that the corresponding region of PKCα should constitute an even better binding site for the Grb-2 SH2 domain (Fig. 2 A) after phosphorylation of Y658 (equivalent to Y662 in PKCβI). To test this possibility, we first examined whether PKCα is phosphorylated on tyrosine in mast cells. As shown in Fig. 3A, FcεRI stimulation induced tyrosine phosphorylation of PKCα in the membrane compartment with similar kinetics observed for PKCβI. Second, similar to PKCβI, tyrosine phosphorylation of PKCα was severely impaired in Syk-deficient RBL-2H3 cells (Fig. 3A). Third, we mapped Y658 as a phosphorylation site by using the same strategy we used for PKCβI (data not shown). Fourth, we showed that the biotinylated C-terminal phosphopeptide (α-C30pSpY), but not the nonphosphorylated (α-C30) or a S657-phosphorylated (α-C30pS) peptide, immobilized onto avidin agarose, precipitates GST-Grb-2 SH2 (Fig. 3B). α-C30pSpY exhibited a higher affinity for the Grb-2 SH2 domain than βI-C25pSpY (data not shown). α-C30pSpY also precipitated Grb-2 from MCP-5 mast cell lysates (data not shown). Fifth, PKCα also coimmunoprecipitated Grb-2 and Sos in activated mast cells (Fig. 3C). These data suggest that PKCα also contributes to the activation of the Ras/ERK pathway. Consistent with this possibility, Gö 6976, a selective inhibitor of the conventional PKC isoforms including PKCα and PKCβI, significantly reduced Ras activation and ERK phosphorylation induced by FcεRI stimulation in wild-type as well as PKCβ-/- BMMCs (Fig. 3D and data not shown). Down-regulation of PKC [induced by chronic treatment of mast cells with phorbol 12-myristate 13-acetate (PMA)] also inhibited FcεRI-induced Ras activation and ERK phosphorylation (Fig. 3D). Because this manipulation reduced the expression of conventional PKCs (data not shown), these results also support the role of PKCα and PKCβI in Ras/ERK activation. Finally, we demonstrated (7) that Gö 6976 inhibits the production and secretion of TNF-α and IL-2 from FcεRI-stimulated BMMCs. Together, these data suggest that Grb-2/Sos binding to plasma membrane-recruited, tyrosine-phosphorylated PKCα and PKCβI contributes to the full activation of the Ras/ERK pathway. This Ras activation pathway may be operational in other immune cells, as PKCβI tyrosine-phosphorylation was observed in anti-IgG-stimulated B cells (data not shown).
Fig. 3.
Tyr-658-phosphorylated PKCα also contributes to the Ras/ERK activation. (A) Wild-type BMMCs were stimulated with IgE and antigen and fractionated into particulate and cytosolic compartments. PKCα immunoprecipitates were analyzed as in Fig. 1 A. Syk-deficient (syk-) and syk-reconstituted syk- (syk+) RBL-2H3 cells were also subjected to similar analysis. (B) N-terminally biotinylated α-C30 peptides, i.e., nonphosphorylated (NP), S657 phosphorylated (pS), and S657/Y658 phosphorylated (pSpY) peptides, which were immobilized onto NeutrAvidin agarose, were incubated with GST, GST-NSH3, or GST-SH2. Bound Grb-2 GST fusion proteins were analyzed by immunoblotting with anti-GST. (C) MCP-5 cells were stimulated with IgE and antigen. Anti-PKCα immunoprecipitates were analyzed by immunoblotting with the indicated antibodies. (D) IgE-sensitized BMMCs were pretreated with 5 μMGö 6976 for 10 min before antigen stimulation (Left). Another set of cells were incubated overnight with 100 nM PMA before antigen stimulation. Cell lysates were processed for the measurement of GTP-bound Ras and ERK phosphorylation as described for Fig. 2 D and E.
Discussion
This study has defined an Syk/PKC-dependent signaling pathway leading to the activation of the Ras/ERK pathway in mast cells. The tyrosine-phosphorylated C terminus of PKCβI recruits Grb-2/Sos complexes to the vicinity of Ras and increases GTP-bound Ras. However, the 25% reduction in Ras activation in PKCβ-/- cells compared with wild-type cells is not a dramatic change. Given the likelihood of redundancy in the PKC isoforms involved in the signal relay pathway we describe, however, this result is not surprising. In this article, we provide clear evidence that tyrosine-phosphorylated PKCα can recruit Grb-2/Sos to activate Ras through a mechanism analogous to tyrosine-phosphorylated PKCβI. The 25% reduction in Ras activation in PKCβ-/- cells also appears to be consistent with the relative amount of conventional PKC isoforms recruited to the membrane fraction. In addition to PKCβI, BMMCs express PKCα, PKCζ, and PKCλ/ι isoforms that are potentially involved in the pathway described. The primary BMMC populations used in this study express ≈40 ng per 106 cells of PKCα, 50 ng of PKCβII, and only 1.5 ng of PKCβI. However, the fraction of the membrane-associated βI isoform (≈10%) during FcεRI stimulation is much higher than that of the membrane-associated α (≈0.5%; Figs. 1 A and 3A). Thus, there are nearly equal concentrations of the PKCβI and PKCα isoforms (≈0.15 ng of PKCβI and 0.2 ng of PKCα) recruited to the membrane after receptor engagement. These considerations suggest that PKCα and PKCβI may contribute to a similar extent to Ras activation through the Grb-2/Sos recruitment. This argument is also further supported by nearly identical levels of Grb-2 associated with PKCα or PKCβI. Among three major classes of PKC, i.e., conventional, novel, and atypical isoforms, the α, βI, γ, ζ, and λ/ι isoforms each contain a potential Syk phosphorylation site that corresponds to Y662 of PKCβI. Y662 (and equivalent tyrosine) phosphorylation by Syk after autophosphorylation of the preceding Ser/Thr residue creates putative Grb-2-binding sites in the conventional PKC isoforms (Fig. 2 A) (PKCγ is a neuron-specific isoform and not expressed in mast cells). On the other hand, potential phosphorylation of the corresponding tyrosine residue of the atypical PKCs by Syk would not involve autophosphorylation of PKC, because the tyrosine residue is preceded by a glutamic acid residue in these isoforms. In our preliminary experiments, we observed tyrosine phosphorylation of PKCζ and PKCλ/ι in FcεRI-stimulated cells (data not shown). Therefore, these atypical isoforms would also be predicted to contribute to Ras-ERK activation. Because of limited similarities in this region, other related kinases of the AGC family (36) are unlikely to play a similar role in Ras activation.
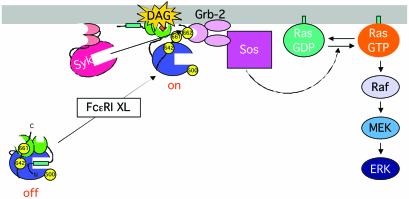
Numerous studies have suggested that PKC mediates Ras/ERK activation. Treatment with phorbol esters and calcium circumvents the requirement for cell-surface receptor engagement, and activates the Ras/ERK pathway. Both PKC-dependent and -independent pathways have been proposed to explain how the engagement of multichain immune receptors activates these pathways: inactivation of RasGAPs by PKC (13) and direct phosphorylation of c-Raf-1 by PKC (14, 15) are potential avenues. Membrane recruitment of the diacylglycerol-binding C1 domain-containing protein, RasGRP, was shown to be a major regulatory mechanism for Ras activation in T cells (16, 17). This GEF protein may contribute to Ras/ERK activation in response to increased levels of diacylglycerol without the involvement of PKCs in other cell types as well [although RasGRP is not expressed in mouse BMMC (data not shown)]. Although our data do not allow direct comparison of the relative roles for alternative routes to Ras/ERK activation, this study clearly demonstrates that Syk-dependent PKCβI/PKCα-mediated recruitment of the Grb-2/Sos complex contributes to the full activation of the Ras/ERK pathway in FcεRI-stimulated mast cells (Fig. 4) and antigen receptor-stimulated B cells (data not shown). The substantial inhibition of Ras/ERK activation exhibited by Gö 6976 and PMA-mediated PKC down-regulation suggests that this may be a major pathway for the Ras/ERK activation in these cells. The incomplete inhibition of ERK activation by these measures is also consistent with a previous report that FcεRI-induced ERK activation depends on both PKC-dependent and -independent components (37). Interestingly, we observed increases in basal Ras activities in mast cells treated with Gö 6976 or chronically with PMA, although not translated to increases in ERK activation (Fig. 3D). This finding may indicate that RasGAP or other GAP proteins are under constitutive inhibition by PKC (13, 38). In the pathway defined in this study, the conventional PKCs primarily play an adaptor function to recruit the Grb-2/Sos complex to the vicinity of Ras (Fig. 4). This adaptor function is exceptional, however, as the enzymatic activity of these PKCs is required: only the species primed by intramolecular autophosphorylation are recognized by Syk.
Fig. 4.
Model for a Ras activation pathway. PKCβI in the cytosol has been phosphorylated at T500, T642, and S661. On FcεRI stimulation, PKCβI is recruited to the plasma membrane by diacylglycerol (DAG) and acid phospholipids, and phosphorylated at Y662 by Syk. The hydrophobic motif with the phosphorylated Y662 now binds Grb-2 by means of the phosphotyrosine–SH2 domain interaction. Grb-2-bound Sos in turn activates Ras. This mechanism can be applicable to PKCα as well.
To the best of our knowledge, this study also represents the first demonstration where a tyrosine residue is phosphorylated by a PTK only after phosphorylation of the preceding serine residue. Because several PTKs prefer Glu-Tyr sequences as part of their recognition sequences (39), we predict that similar phosphorylation targets will be found. This model further underscores the coordinate regulation of signaling events by serine/threonine kinases together with PTKs.
Supplementary Material
Acknowledgments
We thank John Kim for excellent technical help; G. Baier, M. Comb, U. Kikkawa, Y. Ono, and R. P. Siraganian for providing reagents; and M. Ullah for synthesizing peptides. This work was supported by grants from the National Institutes of Health (to T.K., A.C.N., and D.J.R.). This is publication 337 from the La Jolla Institute for Allergy and Immunology.
Abbreviations: PTK, protein tyrosine kinase; FcεRI, high-affinity IgE receptor; SH2, Src homology 2; Btk, Bruton's tyrosine kinase; BMMC, bone marrow-derived mast cell; ERK, extracellular signal-regulated kinase; TNF-α, tumor necrosis factor α.
References
- 1.Weiss, A. & Littman, D. R. (1994) Cell 76, 263-274. [DOI] [PubMed] [Google Scholar]
- 2.Kurosaki, T. (1999) Annu. Rev. Immunol. 17, 555-592. [DOI] [PubMed] [Google Scholar]
- 3.Turner, H. & Kinet, J.-P. (1999) Nature 402, B24-B30. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami, T., Kawakami, Y. & Kitaura, J. (2002) J. Biochem. 132, 677-682. [DOI] [PubMed] [Google Scholar]
- 5.Nishizuka, Y. (1992) Science 258, 607-614. [DOI] [PubMed] [Google Scholar]
- 6.Dekker, L. V. & Parker, P. J. (1994) Trends Biochem. Sci. 19, 73-77. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami, Y., Kitaura, J., Hartman, S. E., Lowell, C. A., Siraganian, R. P. & Kawakami, T. (2000) Proc. Natl. Acad. Sci. USA 97, 7423-7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin, M. (1995) J. Biol. Chem. 270, 16483-16486. [DOI] [PubMed] [Google Scholar]
- 9.Altman, A. & Deckert, M. (1999) Adv. Immunol. 72, 1-101. [DOI] [PubMed] [Google Scholar]
- 10.Hirasawa, N., Scharenberg, A., Yamamura, H., Beaven, M. A. & Kinet, J.-P. (1995) J. Biol. Chem. 270, 10960-10967. [DOI] [PubMed] [Google Scholar]
- 11.Hirasawa, N., Santini, F. & Beaven, M. A. (1995) J. Immunol. 154, 5391-5402. [PubMed] [Google Scholar]
- 12.Boguski, M. S. & McCormick, F. (1993) Nature 366, 643-654. [DOI] [PubMed] [Google Scholar]
- 13.Downward, J., Graves, J. D., Warne, P. H., Rayter, S. & Cantrell, D. A. (1990) Nature 346, 719-723. [DOI] [PubMed] [Google Scholar]
- 14.Siegel, J. N., Klausner, R. D., Rapp, U. R. & Samelson, L. E. (1990) J. Biol. Chem. 265, 18472-18480. [PubMed] [Google Scholar]
- 15.Kolch, W., Heidecker, G., Kochs, G., Hummel, R., Vahidi, H., Mischak, H., Finkenzeller, G., Marme, D. & Rapp, U. R. (1993) Nature 364, 249-252. [DOI] [PubMed] [Google Scholar]
- 16.Ebinu, J. O., Bottorff, D. A., Chan, E. Y., Stang, S. L., Dunn, R. J. & Stone, J. C. (1998) Science 280, 1082-1086. [DOI] [PubMed] [Google Scholar]
- 17.Ebinu, J. O., Stang, S. L., Teixeira, C., Bottorff, D. A., Hooton, J., Blumberg, P. M., Barry, M., Bleakley, R. C., Ostergaard, H. L. & Stone J. C. (2000) Blood 95, 3199-3203. [PubMed] [Google Scholar]
- 18.Khan, W. N., Alt, F. W., Gerstein, R. M., Malynn, B. A., Larsson, I., Rathbun, G., Davidson, L., Mueller, S., Kantor, A. B., Herzenberg, L. A., et al. (1995) Immunity 3, 283-299. [DOI] [PubMed] [Google Scholar]
- 19.Leitges, M., Schmedt, C., Guinamard, R., Davoust, J., Schaal, S., Stabel, S. & Tarakhovsky, A. (1996) Science 273, 788-791. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami, T., Inagaki, N., Takei, M., Fukamachi, H., Coggeshall, K. M., Ishizaka, K. & Ishizaka, T. (1992) J. Immunol. 148, 3515-3519. [PubMed] [Google Scholar]
- 21.Kawakami, Y., Miura, T., Bissonnette, R., Hata, D., Khan, W. N., Kitamura, T., Maeda-Yamamoto, M., Hartman, S. E., Yao, L., Alt, F. W., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 3938-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, J., Berenstein, E. H., Evans, R. L. & Siraganian, R. P. (1996) J. Exp. Med. 184, 71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami, Y., Yao, L., Miura, T., Tsukada, S., Witte, O. N. & Kawakami, T. (1994) Mol. Cell. Biol. 14, 5108-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao, L., Kawakami, Y. & Kawakami, T. (1994) Proc. Natl. Acad. Sci. USA 91, 9175-9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang, S. W., Wahl, M. I., Chu, J., Kitaura, J., Kawakami, Y., Kato, R. M., Tabuchi, R., Tarakhovsky, A., Kawakami, T., Turck, C. W., et al. (2001) EMBO J. 20, 5692-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su, T. T., Guo, B., Kawakami, Y., Sommer, K., Chae, K., Humphries, L. A., Kato, R. M., Kang, S., Patrone, L., Wall, R., et al. (2002) Nat. Immunol. 3, 780-786. [DOI] [PubMed] [Google Scholar]
- 27.Ono, Y., Kikkawa, U., Ogita, K., Fujii, T., Kurokawa, T., Asaoka, Y., Sekiguchi, K., Ase, K., Igarashi, K. & Nishizuka, Y. (1987) Science 236, 1116-1120. [DOI] [PubMed] [Google Scholar]
- 28.Tsutakawa, S. E., Medzihradszky, K. F., Flint, A. J., Burlingame, A. L. & Koshland, D. E., Jr. (1995) J. Biol. Chem. 270, 26807-26812. [DOI] [PubMed] [Google Scholar]
- 29.Keranen, L. M., Dutil, E. M. & Newton, A. C. (1995) Curr. Biol. 5, 1394-1403. [DOI] [PubMed] [Google Scholar]
- 30.Songyang, Z., Blechner, S., Hoagland, N., Hoekstra, M. F., Piwnica-Worms, H. & Cantley, L. C. (1994) Curr. Biol. 4, 973-982. [DOI] [PubMed] [Google Scholar]
- 31.Newton, A. C. (1997) Curr. Opin. Cell Biol. 9, 161-167. [DOI] [PubMed] [Google Scholar]
- 32.Songyang, Z., Shoelson, S. E., Chaudhuri, M., Gish, G., Pawson, T., Haser, W. G., King, F., Roberts, T., Ratnofsky, S., Lechleider, R. J., et al. (1993) Cell 72, 767-778. [DOI] [PubMed] [Google Scholar]
- 33.Buday, L. & Downward, J. (1993) Cell 73, 611-620. [DOI] [PubMed] [Google Scholar]
- 34.Rozakis-Adcock, M., Fernley, R., Wade, J., Pawson, T. & Bowtell, D. (1993) Nature 363, 83-85. [DOI] [PubMed] [Google Scholar]
- 35.Dumitru, C. D., Ceci, J. D., Tsatsanis, C., Kontoyiannis, D., Stamatakis, K., Lin, J. H., Patriotis, C., Jenkins, N. A., Copeland, N. G., Kollias, G., et al. (2000) Cell 103, 1071-1083. [DOI] [PubMed] [Google Scholar]
- 36.Hanks, S. K. & Hunter, T. (1995) in The Protein Kinase FactsBook: Protein-Serine Kinases, eds. Hardie, G. & Hanks, S. (Academic, San Diego), pp. 7-47.
- 37.Zhang, C., Hirasawa, N. & Beaven, M. A. (1997) J. Immunol. 158, 4968-4975. [PubMed] [Google Scholar]
- 38.Schubert, C., Carel, K., DePaolo, D., Leitner, W. & Draznin, B. (1996) J. Biol. Chem. 271, 15311-15314. [DOI] [PubMed] [Google Scholar]
- 39.Songyang, Z., Carraway, K. L., III, Eck, M. J., Harrison, S. C., Feldman, R. A., Mohammadi, M., Schlessinger, J., Hubbard, S. R., Smith, D. P., Eng, C., et al. (1995) Nature 373, 536-539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.