Abstract
Expression of adhesion receptor integrin αvβ3 in an activated functional form strongly promotes metastasis in human breast cancer cells. Here, we report that αvβ3 cooperates with matrix metalloproteinase type 9 (MMP-9) in breast cancer cell migration. This cooperation is regulated by the activation state of the integrin. Expression of activated αvβ3 in metastatic variants of MDA-MB 435 human breast cancer cells and primary metastatic cells from breast cancer patients strongly enhanced migration toward vitronectin and fibrinogen. This enhancement was mediated by a soluble factor produced by breast cancer cells expressing activated αvβ3. When transferred, this factor also up-regulated αvβ3-dependent migration of breast cancer cells that express the nonactivated integrin. The factor was identified as metalloproteinase MMP-9. Whereas all tested breast cancer cell variants produced latent MMP-9, only those with activated αvβ3 produced the mature form of this metalloproteinase. Recombinant mature MMP-9, but not latent MMP-9 or either form of MMP-2, enhanced αvβ3-dependent breast cancer cell migration. The migratory response was inhibited by tissue inhibitors of metalloproteinase or when MMP-9 was depleted from the inducing supernatants. The results indicate a causal relationship between the expression of activated integrin αvβ3 and production of enzymatically active MMP-9 in metastatic breast cancer cells. These molecules cooperate to enhance breast cancer cell migration toward specific matrix proteins, and this may contribute to the strongly enhanced metastatic capacity of breast cancer cells that express activated αvβ3.
Metastasis is the primary cause of death in breast cancer patients. Metastatic dissemination depends on tumor cell adhesion, migration, and invasion. These steps involve integrins, a family of transmembrane adhesion receptors, composed of noncovalently linked α and β subunits (1). Integrins are known to exist in distinct states of activation, and these determine integrin functionality and affinity for ligands (2). For example, integrin activation controls which ligands are recognized, whether an integrin can support cell arrest under dynamic flow conditions or only stationary adhesion, and whether cells can migrate on or toward specific substrates (3). The importance of integrin activation has long been appreciated in leukocytes and platelets, where it controls inflammatory responses and thrombus formation (4, 5). Recent findings indicate that other cell types, such as endothelial cells and tumor cells, can also regulate their interaction with extracellular matrix proteins by integrin activation (6–9). This regulation may help to control angiogenesis and tumor metastasis.
In breast and ovarian cancer, as well as in melanoma and glioma, malignant progression is associated with expression of tumor cell integrin αvβ3 (10–14). We recently found that αvβ3 can exist in human breast cancer cells in an activated or a nonactivated functional state. Only the activated state supports breast cancer cell arrest during blood flow, as in the circulation. Importantly, activated but not nonactivated αvβ3 strongly promotes breast cancer metastasis (9, 15). Whereas tumor cell arrest in flowing blood may be critical during hematogenous metastasis, metastatic dissemination also depends on the control of tumor cell motility. Changes in migratory functions can be induced by growth factors and altered production and activity of matrix-degrading enzymes, such as metalloproteinases. Connections between these phenomena and integrin function are just beginning to emerge (3, 16–18). Here, we asked whether and how the activation state of tumor cell integrin αvβ3 helps to regulate breast cancer cell migration. Our results show that αvβ3 activation strongly enhances breast cancer migration toward specific substrates and that this process depends on a cooperation between the integrin and matrix metalloproteinase type 9 (MMP-9) in an activation-dependent pathway. We thereby establish a mechanism by which the activation state of integrin αvβ3 can contribute to migratory responses of metastatic breast cancer cells.
Methods
Cells. MDA-MB 435 human breast cancer cells were from J. E. Price (M. D. Anderson Cancer Center, Houston). These cells (parent) were injected into the mammary fat pad of immunedeficient mice, and metastatic variants were selected from metastases to bone or lung (9). A β3 integrin negative variant (β3minus) was selected in vitro after treating MDA-MB 435 parent cells with an anti-β3-saporin conjugate (9). The β3-negative variant was stably transfected either with β3 wild type (β3WT) or with constitutively activated mutant β3 (β3D723R) (9). Primary metastatic human breast cancer cells were isolated from a pleural effusion (PEO2JA) (9) or from peripheral blood samples (BCM1, BCM2, and BMS) of stage IV breast cancer patients by immunomagnetic bead sorting with antiepithelial antibody BerEP4 (Dynal, Great Neck, NY). The patient-derived cells express integrin αvβ3 at levels comparable to those of the MDA-MB 435 cell variants. The cells further express EpCAM, an epithelial marker, and mammaglobin.
Migration. Haptotactic breast cancer cell migration toward purified extracellular matrix proteins was analyzed in transwells (8-μm pore size; Costar). Filter undersides (triplicates) were coated with human vitronectin (10 μg/ml), fibrinogen (plasminogen free) (20 μg/ml), von Willebrand factor (5 g/ml), or BSA in PBS and blocked with 2.5% BSA in 0.2% Tween 20/PBS. Cells were starved overnight in serum-free medium (Eagle's minimal essential medium, EMEM), harvested with PBS/EDTA, washed, and seeded at 3 × 104 cells per upper transwell. After 16 h, or longer if indicated, at 37°C, 5% CO2, filters were washed, and cells from the filter tops were removed, fixed, and stained (DiffQuick). Migrated cells were counted in five random microscopic fields per filter. Migration medium was serum-free EMEM or conditioned supernatants of breast cancer cell variants that were collected from 19-h cultures in serum-free EMEM and centrifuged before use. Inhibitors were added to cells immediately before the migration assay and left in place for the duration of the test [function blocking anti-β3 integrin mAb 7E3 at 80 μg/ml, tissue inhibitors of metalloproteinase-1 (TIMP-1) at 1 μg/ml, TIMP-2 at 3.75 μg/ml]. Latent or mature recombinant human MMP-2 or MMP-9 (Oncogene; 40 ng/ml) were added to serum-free EMEM migration medium or were used at this concentration to preincubate cells or matrix proteins as soluble of immobilized substrates before use in migration assays. Pairwise differences were evaluated by standard analyses of variance followed by multiple comparisons (Scheffé's method).
Zymography. Metalloproteinase activity in supernatants was tested by zymography. Conditioned media were harvested from 48-h cultures in serum-free EMEM, and proteins were precipitated at 56% (NH4)2SO4 overnight at 4°C, pelleted at 19,000 × g, and dialyzed against TBS containing 0.0005% Brij35. Protein concentrations were determined by MicroBCA (Pierce). Twenty-five micrograms of total protein were analyzed under nonreducing conditions on 8% polyacrylamide gels containing either 0.05% gelatin or 0.005% human fibrinogen. For development, gels were washed three times in 2.5% Triton X-100, incubated overnight at 37°C in 40 mM Tris/200 mM NaCl/10 mM CaCl2 (pH 7.5), and stained with Coomassie blue.
Immunodepletion. MMP-9 was depleted from conditioned breast cancer cell supernatants or EMEM containing 40 ng/ml human recombinant mature MMP-9 by three rounds of incubation (45 min each at 22°C) with protein A-Sepharose beads (Repligen) carrying rabbit anti-mouse IgG (Zymed) and monoclonal murine anti-human MMP-9 antibody (Oncogene Ab-7). Control supernatants were incubated three times with protein-A anti-mouse beads without anti-MMP-9.
Results
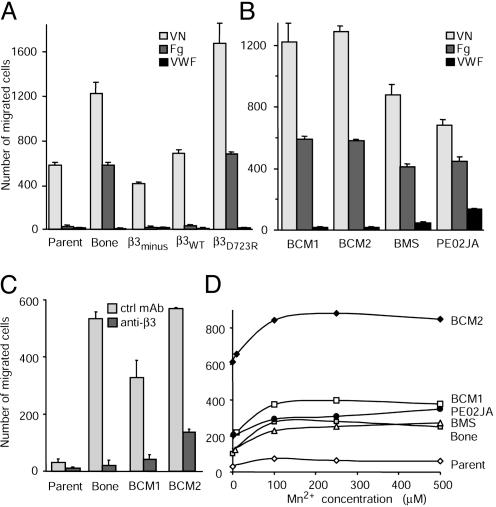
Activated Integrin αvβ3 Enhances Matrix-Directed Breast Cancer Cell Migration. Expression of integrin αvβ3 in a constitutively activated functional form strongly enhances metastasis of human breast cancer cells (9). To analyze mechanisms through which the activated receptor may support metastatic dissemination, we compared the migratory activities of human breast cancer cell variants that stably express αvβ3 either in an activated or a nonactivated functional form. The MDA-MB 435 parental cell population at large expresses nonactivated αvβ3, whereas its in vivo-selected, metastasis-derived variants express activated αvβ3 (9). These cells were compared with an in vitro selected β3-lacking MDA-MB 435 variant (β3minus) or its transfectants that express either nonactivated αvβ3 (β3WT) or constitutively activated mutant β3 (β3D723R). Breast cancer cell variants with activated αvβ3 showed enhanced migration toward vitronectin and fibrinogen (Fig. 1A). This finding has clinical relevance because primary metastatic cells from stage IV breast cancer patients, isolated either from a pleural effusion (PE02JA) or from peripheral blood samples (BCM1, BCM2, and BMS) express activated αvβ3 (Table 1). The activated receptor supports platelet-mediated tumor cell arrest during blood flow (9) and mediated enhanced migration toward vitronectin and fibrinogen compared with MDA-MB 435 parental cells, which express αvβ3 at a similar level but in a nonactivated functional form (Fig. 1B). Whereas vitronectin-directed migration was supported by αvβ3 plus other αv integrins, fibrinogen-directed migration depended on αvβ3, as it was strongly inhibited by a function blocking anti-β3 antibody (Fig. 1C). Thus, migration of metastatic breast cancer cells toward fibrinogen is mediated by integrin αvβ3, and it is very strongly enhanced if the receptor is activated. Exogenous integrin activation of MDA-MB 435 parental cells with Mn2+ did not significantly enhance migration (Fig. 1D). This indicates that breast cancer cell migration depends on the endogenous control of αvβ3 functionality and perhaps on other supporting factors.
Fig. 1.
Activated integrin αvβ3 enhances matrix-directed breast cancer cell migration. (A) Migration of MDA-MB 435 human breast cancer cells (Parent), their in vivo-selected metastatic variant from a bone metastase in the SCID mouse model (Bone), or their in vitro generated variants that lack β3 integrin expression (β3minus) were transfected to express either the β3 wild-type gene (β3WT) or the constitutively activated mutant β3D723R toward vitronectin (VN), fibrinogen (Fg), or von Willebrand factor (VWF) in transwell chambers (16 h at 37°C and 5% CO2). Values are mean numbers of cells in five optical fields ± SD counted at the filter underside after staining with DiffQuick. (B) Migration of primary metastatic cells from breast cancer patients (BCM1, BCM2, and BMS captured from blood samples with immunomagnetic beads carrying antiepithelial mAb BerEP4, and PE02JA isolated from a pleural effusion). The cells were established in culture and used at early passages. Migration conditions were as described above. (C) Fibrinogen-directed migration of MDA-MB 435 parental cells (Parent) or their metastasis-derived variant (Bone), BCM1, and BCM2 cells in the presence of function-blocking anti-β3 mAb 7E3 or nonfunction-blocking anti-β3 mAb AV10 (80 μg/ml). (D) Effect of Mn2+ treatment on fibrinogen-directed breast cancer cell migration. Fibrinogen-directed migration of MDA-MB 435 parental cells (Parent), their metastatic variant (Bone), or patient-derived metastatic breast cancer cells in the presence of increasing Mn2+ concentrations is shown. All other assays in this study were done without addition of Mn2+.
Table 1. Expression of total versus activated integrin αvβ3 in the used breast cancer cell variants.
| αvβ3 | Parent | Bone | β3minus | β3WT | β3D723R | BCM1 | BCM2 | BMS |
|---|---|---|---|---|---|---|---|---|
| Total | 89.54 | 95.46 | 5.63 | 68.75 | 78.34 | 96.42 | 99.03 | 97.29 |
| Activated | 15.68 | 69.73 | 0.05 | 11.45 | 73.03 | 85.92 | 97.08 | 89.41 |
αvβ3 expression measured by flow cytometry [mAb LM609 reports total αvβ3, and mAb WOW-1 (46) reports activated αvβ3]. Values are percent positive cells within the total cell populations. Specific LM609 binding was determined by using an IgG1 isotype control, and specific WOW-1 binding was defined as that inhibited by 2 mM RGDS peptide. WOW-1 binding was tested in the presence of Ca2+/Mg2+ as detailed (9, 46). Parent, bone, and the β3 variants are derivatives of the MDA-MB 435 cell line. BCM1, BCM2, and BMS are primary metastatic cells isolated from blood samples of stage IV breast cancer patients
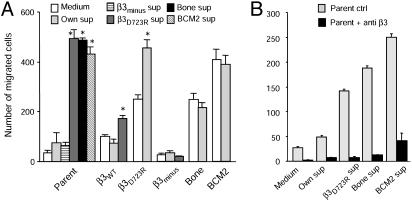
Breast Cancer Cells with Activated αvβ3 Produce a Migration-Stimulating Factor. To probe for potential factors that are produced by activated αvβ3 expressing breast cancer cells and modify their migration, we analyzed conditioned supernatants of these cells. Supernatants of MDA-MB 435 bone cells and the β3D723R expressing variant, as well as patient-derived metastatic breast cancer cells, induced fibrinogen-directed migration of MDA-MB 435 parental cells (Fig. 2A). This was mediated by integrin αvβ3 of the responding cells (Fig. 2B). Conditioned supernatants of the in vivo selected metastatic cell variant (bone) and primary metastatic cells from breast cancer patients (BCM2) did not further increase their own robust fibrinogen-directed migration, but supernatants of the in vitro generated MDA-MB 435 variant β3D723R maximized the migratory response in these cells (Fig. 2 A). Importantly, supernatants of β3D723R-expressing cells or primary metastatic breast cancer cells induced fibrinogen-directed migration in the β3WT-expressing cell variant, but not in β3minus cells (Fig. 2 A). The promigratory effect was specific for the haptotactic substrate, because it was seen on fibrinogen and to a lesser extent on fibronectin, but not on vitronectin. Conditioned supernatants of MDA-MB 435 parental cells or their β3minus- or β3WT-expressing variants did not stimulate fibrinogen-directed migration (Fig. 2 A). This indicates that only breast cancer cells with activated αvβ3 produce and release a soluble factor(s), which stimulates αvβ3-mediated, substrate-specific migration in breast cancer cells, even if they per se express the nonactivated receptor.
Fig. 2.
Breast cancer cells expressing activated integrin αvβ3 produce a factor that stimulates αvβ3-mediated migration. (A) Supernatants from breast cancer cells expressing activated αvβ3 enhance migration. Fibrinogen-directed migration of MDA-MB 435 parental cells (Parent), their metastasis-derived variant (Bone), or their in vitro generated variants expressing β3D723R or lacking β3 integrin (β3minus) and patient-derived BCM2 cells. Migration in serum-free medium or conditioned supernatants from 19-h cell cultures in serum-free medium. *, P < 0.05, significantly enhanced over medium only. Marked on the abscissa are cell types used for migration; the key shows cell types used for supernatant production. (B) Supernatant-induced migration is mediated by αvβ3 of the responding cells. Fibrinogen-directed migration of MDA-MB 435 parental cells (Parent) in medium or conditioned supernatants in the presence of nonfunction blocking anti-β3 mAb AV-10 (ctrl, gray) or function-blocking anti-β3 mAb 7E3 (black) is shown. Marked on the abscissa are the types of medium or spent supernatants used as migration buffer.
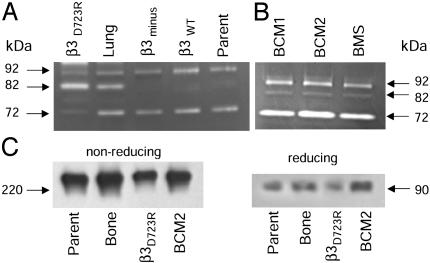
Breast Cancer Cells with Activated αvβ3 Produce the Mature Form of Metalloproteinase MMP-9. Compounds known to alter tumor cell migration are the metalloproteinases (19). Therefore, we analyzed metalloproteinases in conditioned supernatants of breast cancer cell variants that express either activated or nonactivated αvβ3. All tested variants of the MDA-MB 435 breast cancer cell line produced a gelatinase with an apparent molecular mass of 92 kDa (Fig. 3A). This is consistent with characteristics of latent metalloproteinase MMP-9 (proMMP-9). The presence of MMP-9 was confirmed by Western analysis (Fig. 3C). Interestingly, an 82-kDa gelatinase consistent with mature MMP-9 was detected only in supernatants of breast cancer cells that express activated αvβ3 (MDA-MB 435 lung and β3D723R shown in Fig. 3A on a gelatin zymogram, and BCM1, BCM2, and BMS shown in Fig. 3B on a fibrinogen zymogram). In addition, all breast cancer cell variants produced a 72-kDa gelatinase, most likely MMP-2, regardless of their αvβ3 activation state. The presence of MMP-2 was confirmed by Western analysis. Thus, both groups of breast cancer cells, those with activated and those with nonactivated integrin αvβ3, produce proMMP-2 and proMMP-9. However, production of mature MMP-9 seems to be restricted to cells expressing activated αvβ3. Thus, enhanced αvβ3-dependent breast cancer cell migration toward fibrinogen coincides with the ability of the cells to generate a metalloproteinase with characteristics of mature MMP-9.
Fig. 3.
Breast cancer cells expressing activated αvβ3 produce a gelatinolytic and fibrinogenolytic 82-kDa metalloproteinase. (A and B) Zymography of secreted proteins from MDA-MB 435 cell variants or primary metastatic breast cancer cells. Secreted proteins were precipitated from spent supernatants of the parental cells (Parent), their metastasis-derived variant (Lung), or the in vitro generated variants that lack β3 integrin (β3minus) or express either activated mutant βD723R or nonactivated β3WT. BCM1, BCM2, and BMS are patient-derived primary metastatic cells. Twenty-five micrograms of total protein were separated on polyacrylamide gels (8%) containing 0.05% gelatin (A) or 0.005% human fibrinogen (B) (nonreducing). (C) Detection of MMP-9 in breast cancer cell supernatants and Western blot analysis of concentrated spent supernatants after SDS/PAGE (8%) separation under nonreducing (Left) or reducing (Right) conditions. Reactivity is shown with anti-MMP-9 (oncogene mAb-7). The nonreduced 240-kDa bands were also positive with anti-TIMP-1 (oncogene mAb-1), consistent with an MMP-9/TIMP-1 complex dimer.
MMP-9 may affect fibrinogen-directed breast cancer cell migration by modifying the substrate and converting it into a promigratory format (20–22). The 82-kDa metalloproteinase produced by breast cancer cells expressing activated αvβ3 degraded fibrinogen, as shown by zymography (Fig. 3B). This indicates that breast cancer cells with activated αvβ3, but not those with nonactivated αvβ3, produce a metalloproteinase whose apparent molecular weight, substrate specificity, and immunologic reactivity match that of mature MMP-9. The mature enzyme may enhance fibrinogen-directed tumor cell migration.
MMP-9 Stimulates αvβ3-Dependent Breast Cancer Cell Migration. The production of mature MMP-9 was found restricted to breast cancer cells with activated αvβ3, and only supernatants of these cells promoted αvβ3-dependent migration. To test directly whether mature MMP-9 is responsible for this effect, we analyzed whether the effect was inhibited by MMP antagonists, mimicked by recombinant mature MMP-9, and reversed by depleting MMP-9 from the supernatants.
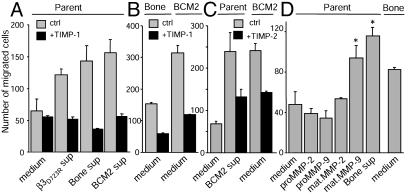
To test whether MMP antagonists block the promigratory effect of supernatants from activated αvβ3-expressing breast cancer cells, MDA-MB 435 parental cells were allowed to migrate toward fibrinogen in conditioned supernatants of MDA-MB 435 bone or BCM2 cells, in the presence or absence of TIMP. The promigratory effect of the supernatants was abolished by TIMP-1 (1 μg/ml, Fig. 4 A and B) and reduced by TIMP-2 (3.75 μg/ml, Fig. 4C). This indicates that a metalloproteinase stimulates αvβ3-dependent, substrate-specific breast cancer cell migration. Importantly, this metalloproteinase is produced by metastatic breast cancer cells with activated αvβ3 and maximizes their αvβ3-mediated migration.
Fig. 4.
MMP-9 stimulates breast cancer cell migration. (A–C) Fibrinogen-directed migration of MDA-MB 435 parental cells (Parent), their metastasis-derived variant (Bone), and patient-derived BCM2 cells is inhibited by TIMP-1 and TIMP-2. (A) Parent cells in medium or spent supernatants from cells expressing activated αvβ3 in the absence (gray) or presence (black) of metalloproteinase inhibitor TIMP-1 (1 μg/ml). (B) Bone and BCM2 cells in medium with or without 1 μg/ml TIMP-1. (C) Parent or BCM2 cells in medium or supernatant from BCM2 cells with or without 3.75 μg/ml TIMP-2. (D) Recombinant mature MMP-9 promotes breast cancer cell migration. Fibrinogen-directed parent cell migration in medium with our without added recombinant forms of human MMP-2 or MMP-9 (40 ng/ml), or in spent supernatant from bone cells compared with bone cell migration in medium. *, P < 0.05, significantly greater than parent cell migration at any other condition in D.
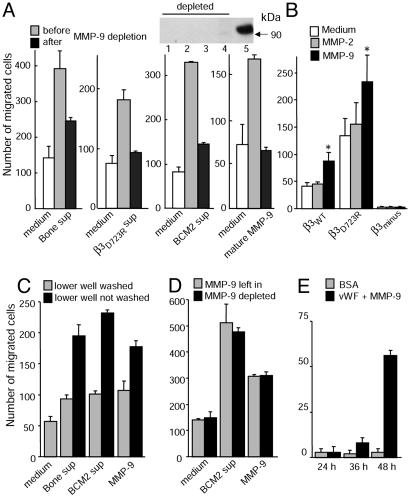
To analyze whether MMP-9 can stimulate αvβ3-mediated breast cancer cell migration toward fibrinogen, MDA-MB 435 parental cells were allowed to migrate in plain medium supplemented with recombinant mature MMP-9, compared with proMMP-9 or either form of MMP-2. Whereas MMP-2 and proMMP-9 had no effect, mature MMP-9 increased migration of the parental cells to levels seen in conditioned supernatants of the bone metastasis-derived cell variant (Fig. 4D). Thus, purified mature MMP-9 and supernatants produced by activated αvβ3-expressing breast cancer cells have similar effects. Both stimulated αvβ3-dependent breast cancer cell migration. To prove that MMP-9 is indeed the active component in conditioned supernatants of metastatic breast cancer cells that maximizes fibrinogen-directed migration, MMP-9 was depleted from these supernatants. The promigratory effects of supernatants from breast cancer cells with activated αvβ3 (MDA-MB 435 bone or β3D723R and BCM2) were strongly decreased when MMP-9 was removed from the supernatants. Similarly, depletion of MMP-9 from MMP-9-spiked medium reversed the promigratory effect (Fig. 5A). Control treatment of conditioned supernatants with protein-A anti-mouse beads without anti-MMP-9 did not compromise the promigratory effect (Fig. 5A). Therefore, the mature form of MMP-9, produced by metastatic breast cancer cells, maximizes αvβ3-dependent breast cancer cell migration toward a fibrinogen substrate. The promigratory effect of MMP-9 was also observed when αvβ3-positive breast cancer cells were pretreated with mature MMP-9 and washed before the migration assay (Fig. 5B). Treatment of β3-lacking breast cancer cells had no effect, as did treatment of either cell variant with MMP-2. Thus, MMP-9 can enhance αvβ3-mediated breast cancer cell migration toward fibrinogen through modification of the cell surface. In addition, MMP-9 produced by breast cancer cells expressing activated αvβ3 can degrade fibrinogen (Fig. 3B) and may convert it into a promigratory format. To test this, immobilized or soluble fibrinogen was digested with spent supernatants of breast cancer cells expressing activated αvβ3 (MDA-MB 435 bone or BCM2) or with recombinant mature MMP-9. Digestion of fibrinogen matrices (Fig. 5C) or soluble fibrinogen (Fig. 5D) enhanced migration of breast cancer cells that express nonactivated αvβ3 (MDA-MB 435 parent), especially when the fibrinogen degradation products remained in the lower transwell or were coated to the underside of the filters. This was observed with and without depletion of MMP-9 from the digests (Fig. 5D). Prolonged exposure of von Willebrand factor to MMP-9 or breast cancer cells, which express activated αvβ3 and release mature MMP-9, eventually triggered some migration. The delay in the migratory response may be caused by the complex and highly multimeric nature of this substrate (Fig. 5E).
Fig. 5.
Breast cancer cells expressing activated αvβ3 produce MMP-9 as a promigratory factor. (A) MMP-9-depleted supernatants from activated αvβ3-expressing breast cancer cells lose their promigratory effect. Fibrinogen-directed migration of MDA-MB 435 parental cells in medium (white) and spent supernatants of breast cancer cell variants expressing activated αvβ3 or medium plus 40 ng/ml recombinant mature MMP-9 before (gray) or after (black) immunodepletion of MMP-9 are shown. Controls (gray) were treated three times with protein A anti-mouse beads; depleted samples (black) were treated three times with protein A anti-mouse/anti-MMP-9 beads. Abscissa notations, cell types from which the supernatants were taken. (Inset) Western blot analysis of MMP-9-depleted supernatants of bone (1), β3D723R-expressing cells (2), BCM2 cells (3), or MMP-9 spiked medium (4) compared with control-treated medium containing MMP-9 (5). (B) Recombinant MMP-9 enhances migration of αvβ3-positive breast cancer cells. Fibrinogen-directed migration of MDA-MB 435 cell variants expressing β3WT or constitutively activated mutant β3D723R, or which lack β3 (β3minus), pretreated for 2 h with medium (white) or medium plus 40 ng/ml recombinant mature MMP-2 (gray) or MMP-9 (black) and washed before the migration assay. *, P < 0.05, significantly greater than both other conditions for these cell types. (C and D) MMP-9-digested fibrinogen is strongly haptotactic. (C) Digestion of a fibrinogen matrix. MDA-MB 435 parent cell migration toward modified fibrinogen matrices. Undersides of transwell filters were coated with 20 μg/ml fibrinogen, BSA blocked, and then placed in fresh lower wells with plain medium and exposed to spent supernatants from MDA-MB 435 bone or BCM2 cells, or to medium containing 40 ng/ml recombinant mature MMP-9 in the upper well for 4 h at 37°C. Before adding cells, upper wells were washed, and the liquid in the lower wells was replaced by fresh plain medium (gray) or kept in place (black). (D) Digestion of soluble fibrinogen. MDA-MB 435 parent cell migration toward digested fibrinogen is shown. Soluble fibrinogen (20 μg/ml) was digested with spent supernatant of BCM2 cells or with 40 ng/ml recombinant mature MMP-9 for4hat37°C, then treated three times either with protein A anti-mouse beads (gray) or protein A anti-mouse/anti-MMP-9 to deplete MMP-9 (black), before coating transwell filters with the digested material. (E) Delayed migration toward von Willebrand factor, which was digested with MMP-9 for 13 h at 37°C, then coated to filters and blocked; BCM2 cells were then allowed to migrate in EMEM for up to 48 h.
Together, breast cancer cells expressing activated integrin αvβ3 are highly metastatic and migrate much more actively toward certain matrix proteins than their nonactivated αvβ3-expressing counterparts. The enhanced migration is mediated through a cooperation between integrin αvβ3 and the mature form of metalloproteinase MMP-9. Expression of activated αvβ3 and production of mature MMP-9 coincide in metastatic breast cancer cells and maximize αvβ3-mediated migration in a substrate-specific manner.
Discussion
Breast cancer cells can express the adhesion receptor integrin αvβ3 in an activated or a nonactivated functional form. Expression of activated αvβ3 promotes tumor cell arrest during blood flow and causes a drastic increase in metastatic activity (9, 15, 23). Integrin activation modulates ligand recognition, cell migration, and invasion (6, 7, 24–26) and endows tumor cells with crucial functions that permit or facilitate a disseminating phenotype. Here, we analyzed consequences of αvβ3 activation on breast cancer cell motility, an important function during metastasis. We show that intrinsic αvβ3 activation strongly increased breast cancer cell migration, involving a mechanism where αvβ3 cooperates with metalloproteinase MMP-9 to maximize tumor cell motility.
To analyze specific contributions of activated versus nonactivated integrin αvβ3, we chose a cell model based on MDA-MB 435 human breast cancer cells. In vivo selected metastatic variants of this cell line express activated αvβ3 (9). This was recapitulated in vitro by transfecting a β3 minus variant of these cells with constitutively activating mutant β3D723R, which caused a drastic increase in metastatic activity compared with β3WT (9). To analyze a clinical significance of αvβ3 activation, we established primary metastatic cells from blood samples of stage IV breast cancer patients. These cells express integrin αvβ3 in an activated functional form as the receptor supports tumor cell arrest during blood flow (9, 23). In these cell models, αvβ3 activation caused enhanced breast cancer cell migration toward fibronectin (9), vitronectin, and fibrinogen. Integrin activation may alter ligand specificity and induce migration on or toward matrix proteins that are not recognized as migratory substrates by the nonactivated receptors (6, 24, 27, 28). Vitronectin and fibronectin are recognized by breast cancer cell integrin αvβ3 plus other αv and β1 integrins, whereas fibrinogen is recognized by αvβ3 only. Migration toward fibrinogen was poor in breast cancer cells with nonactivated αvβ3 but very strong in cells with activated αvβ3. Therefore, we focused on fibrinogen to analyze how breast cancer cell motility is affected by αvβ3 activation.
We found that breast cancer cells can up-regulate αvβ3-mediated migration through cooperation of αvβ3 with metalloproteinase MMP-9 in an activation-dependent pathway. Expression and ligation of αvβ3 and other integrins can stimulate MMP-9 production and function (29–31). However, we show that the presence of αvβ3 is not sufficient to up-regulate MMP-9 functionality, but that this is regulated by the activation state of the integrin. In our breast cancer cell model, all tested variants produced proMMP-9, but only cells expressing activated αvβ3 released a metalloproteinase with characteristics of mature MMP-9. This compound up-regulated αvβ3-mediated migration of breast cancer cells very strongly, even if these per se express nonactivated αvβ3 (cis- and trans-activation). The promigratory factor in supernatants of breast cancer cells with activated αvβ3 was identified as mature MMP-9 by its apparent molecular weight, substrate specificity, inhibition by its natural inhibitors, and immunoreactivity/depletion with monoclonal antibodies. Expression of activated αvβ3 and release of mature MMP-9 coincided in the in vivo selected metastatic MDA-MB 435 cell variants and in patient-derived metastatic breast cancer cells. The finding that the in vitro generated MDA-MB 435 variant β3D7223R also produced mature MMP-9 implies a causal relationship between αvβ3 activation and MMP-9 maturation. These cells were generated by transfecting MDA-MB 435 β3minus cells with β3D723R, which carries a mutation in the cytoplasmic β3 hinge region and renders αvβ3 constitutively active (32, 33). β3D723R-expressing cells are highly metastatic, produce mature MMP-9, and migrate actively toward fibrinogen. Transfection of MDA-MB 435 β3minus cells with β3WT, instead of β3D723R, resulted in a variant that expresses nonactivated αvβ3, metastasizes very poorly (9), does not produce mature MMP-9, and largely fails to migrate toward fibrinogen. Thus, in this cell model, MMP-9 maturation and its consequences for cell migration seem to be directly linked to the activation state of integrin αvβ3.
A mechanism by which activated integrin αvβ3 may contribute to MMP-9 maturation is not known. MMPs are secreted by stromal or tumor cells as inactive pro-enzymes and must bind to the cell surface, where they are processed and activated (34). For MMP-2, this is mediated by an MT1-MMP/TIMP-2/MMP-2 ternary complex or an alternative protein C-dependent pathway (34, 35). It may involve integrin αvβ3 on sprouting endothelial cells, where αvβ3 recognizes the C-terminal hemopexin homology domain of MMP-2 (36) and colocalizes with MMP-2 at the invasive cell front (34). Disrupting αvβ3/MMP-2 binding inhibits angiogenesis (36–38). A mechanism for MMP-9 activation at the cell surface has yet to be established. Cell-surface molecules known to bind MMP-9 are the α2(IV) chain of collagen IV (39) and the hyaluronan receptor CD44 (40). Our results indicate that tumor cell αvβ3 could be involved in MMP-9 processing and targeting the enzyme to cell-matrix contacts. Human MMP-9 contains an RGD sequence at position 366–368 within the third fibronectin-type II domain (41). This site may serve as recognition motif for αvβ3. The RGD motif is absent in MMP-2 (42). As proMMP-9 is secreted, its binding as a soluble ligand would likely require αvβ3 activation. This may explain why we found mature MMP-9 only in supernatants of breast cancer cells expressing activated αvβ3. Our cell model will be useful to test whether αvβ3 and its activation state are directly involved in MMP-9 processing.
The promigratory effect of mature MMP-9 was substrate specific and very pronounced in fibrinogen-directed migration. Fibrinogen is degraded by MMP-9 (20–22). As a result, integrin-mediated interaction may switch from support of firm adhesion to dynamic migration on the modified substrate (17). Substrate processing by highly aggressive tumor cells can alter the response of less aggressive tumor cells in the vicinity (43). Fibrinogen fragments released by MMP-9 may further attract breast cancer cells, as they are potent chemotaxins for neutrophils (44). In addition, MMP-9 may exert a promigratory effect by modifying the functionality of breast cancer cell integrin αvβ3. Metalloproteinases can promote tumor cell migration by modifying integrin subunits, thereby altering the activation state of the receptor (26, 45). In our model, we preincubated nonactivated αvβ3-expressing breast cancer cells with MMP-9 or supernatants of cells bearing the activated receptor. This enhanced αvβ3-dependent migration. Thus, it is possible that the promigratory effect of MMP-9 was mediated through modification of the matrix as well as alterations of the cell surface.
Fibrinogen and fibrin are relevant matrices that disseminating breast cancer cells may encounter during metastasis. These proteins are found in primary tumor stroma, in the circulation, at sites of extravasation, and in the parenchyma of target organs where permeability factors cause fibrinogen leakage from the vasculature. A cascade of events, which begins with intrinsic activation of breast cancer cell integrin αvβ3, may therefore trigger a series of responses that lead to an increased propensity of tumor cells to interact with their matrices, arrest within the vasculature, and migrate toward specific substrates. Our study indicates that this can be potentiated by a positive feedback pathway where intrinsic activation of tumor cell αvβ3 upregulates metalloproteinase functionality, and this in turn enhances αvβ3 activity on neighboring tumor cells and converts matrix proteins into promigratory substrates.
Acknowledgments
We thank Drs. S. J. Shattil and J. A. Koziol (The Scripps Research Institute) for mAb WOW-1 and help with statistical analyses, and Dr. W. Zieger (Frauenklinik, University of Mannheim, Mannheim, Germany) for supporting M.R. This work was supported in part by National Institutes of Health Grants R01 CA95458 (to B.F.-H.) and M01 RR00833 (to The Scripps General Clinical Research Center), U.S. Army Breast Cancer Research Program Grant DAMD 17-99-1-9368 (to B.F.-H.), and Deutsche Forschungsgemeinschaft Fellowships Ro 2363 (to M.R.) and Pi 402 (to J.P.). This is Scripps Research Institute manuscript no. 15306-MEM.
Abbreviations: TIMP, tissue inhibitor of metalloproteinase; EMEM, Eagle's minimal essential medium; MMP-9, matrix metalloproteinase type 9.
References
- 1.Ruoslahti, E. (1999) Adv. Cancer Res. 76, 1-20. [DOI] [PubMed] [Google Scholar]
- 2.Humphries, M. J. (2002) Arthritis Res. 4, Suppl. 3, S69-S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz, M. A. & Ginsberg, M. H. (2002) Nat. Cell Biol. 4, E65-E68. [DOI] [PubMed] [Google Scholar]
- 4.Altieri, D. C. (1999) Thromb. Haemostasis 82, 781-786. [PubMed] [Google Scholar]
- 5.Savage, B., Almus-Jacobs, F. & Ruggeri, Z. M. (1998) Cell 94, 657-666. [DOI] [PubMed] [Google Scholar]
- 6.Byzova, T. V. & Plow, E. F. (1998) J. Cell Biol. 143, 2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byzova, T. V., Kim, W., Midura, R. J. & Plow, E. F. (2000) Exp. Cell Res. 254, 299-308. [DOI] [PubMed] [Google Scholar]
- 8.Dormond, O., Foletti, A., Paroz, C. & Ruegg, C. (2001) Nat. Med. 7, 1041-1047. [DOI] [PubMed] [Google Scholar]
- 9.Felding-Habermann, B., O'Toole, T. E., Smith, J. W., Fransvea, E., Ruggeri, Z. M., Ginsberg, M. H., Hughes, P. E., Pampori, N., Shattil, S. J., Saven, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carreiras, F., Denoux, Y., Staedel, C., Lehmann, M., Sichel, F. & Gauduchon, P. (1996) Gynecol. Oncol. 62, 260-267. [DOI] [PubMed] [Google Scholar]
- 11.Albelda, S. M., Mette, S. A., Elder, D. E., Stewart, R., Damjanovich, L., Herlyn, M. & Buck, C. A. (1990) Cancer Res. 50, 6757-6764. [PubMed] [Google Scholar]
- 12.Natali, P. G., Hamby, C. V., Felding-Habermann, B., Liang, B., Nicotra, M. R., Di Filippo, F., Giannarelli, D., Temponi, M. & Ferrone, S. (1997) Cancer Res. 57, 1554-1560. [PubMed] [Google Scholar]
- 13.Gingras, M. C., Roussel, E., Bruner, J. M., Branch, C. D. & Moser, R. P. (1995) J. Neuroimmunol. 57, 143-153. [DOI] [PubMed] [Google Scholar]
- 14.Pignatelli, M., Cardillo, M. R., Hanby, A. & Stamp, G. W. (1992) Hum. Pathol. 23, 1159-1166. [DOI] [PubMed] [Google Scholar]
- 15.Pilch, J., Habermann, R. & Felding-Habermann, B. (2002) J. Biol. Chem. 277, 21930-21938. [DOI] [PubMed] [Google Scholar]
- 16.Eccles, S. A. (2000) Cancer Res. 157, 41-54. [DOI] [PubMed] [Google Scholar]
- 17.Giannelli, G., Falk-Marzillier, J., Schiraldi, O., Stetler-Stevenson, W. G. & Quaranta, V. (1997) Science 277, 225-228. [DOI] [PubMed] [Google Scholar]
- 18.Byzova, T. V., Goldman, C. K., Pampori, N., Thomas, K. A., Bett, A., Shattil, S. J. & Plow, E. F. (2000) Mol. Cell 6, 851-860. [PubMed] [Google Scholar]
- 19.Stetler-Stevenson, W. G. & Yu, A. E. (2001) Semin. Cancer Biol. 11, 143-152. [DOI] [PubMed] [Google Scholar]
- 20.Lyons, J. G., Birkedal-Hansen, B., Moore, W. G., O'Grady, R. L. & Birkedal-Hansen, H. (1991) Biochemistry 30, 1449-1456. [DOI] [PubMed] [Google Scholar]
- 21.Bini, A., Wu, D., Schnuer, J. & Kudryk, B. J. (1999) Biochemistry 38, 13928-13936. [DOI] [PubMed] [Google Scholar]
- 22.Hiller, O., Lichte, A., Oberpichler, A., Kocourek, A. & Tschesche, H. (2000) J. Biol. Chem. 275, 33008-33013. [DOI] [PubMed] [Google Scholar]
- 23.Felding-Habermann, B., Habermann, R., Saldivar, E. & Ruggeri, Z. M. (1996) J. Biol. Chem. 271, 5892-5900. [DOI] [PubMed] [Google Scholar]
- 24.Garcia, A. J., Schwarzbauer, J. E. & Boettiger, D. (2002) Biochemistry 41, 9063-9069. [DOI] [PubMed] [Google Scholar]
- 25.Kiosses, W. B., Shattil, S. J., Pampori, N. & Schwartz, M. A. (2001) Nat. Cell Biol. 3, 316-320. [DOI] [PubMed] [Google Scholar]
- 26.Ratnikov, B. I., Rozanov, D. V., Postnova, T. I., Baciu, P. G., Zhang, H., DiScipio, R. G., Chestukhina, G. G., Smith, J. W., Deryugina, E. I. & Strongin, A. Y. (2002) J. Biol. Chem. 277, 7377-7385. [DOI] [PubMed] [Google Scholar]
- 27.Faccio, R., Grano, M., Colucci, S., Zallone, A. Z., Quaranta, V. & Pelletier, A. J. (1998) Biochem. Biophys. Res. Commun. 249, 522-525. [DOI] [PubMed] [Google Scholar]
- 28.Faccio, R., Grano, M., Colucci, S., Villa, A., Giannelli, G., Quaranta, V. & Zallone, A. (2002) J. Cell Sci. 115, 2919-2929. [DOI] [PubMed] [Google Scholar]
- 29.Bendeck, M. P., Irvin, C., Reidy, M., Smith, L., Mulholland, D., Horton, M. & Giachelli, C. M. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1467-1472. [DOI] [PubMed] [Google Scholar]
- 30.Thomas, G. J., Poomsawat, S., Lewis, M. P., Hart, I. R., Speight, P. M. & Marshall, J. F. (2001) J. Invest. Dermatol. 116, 898-904. [DOI] [PubMed] [Google Scholar]
- 31.Vacca, A., Ribatti, D., DiRaimondo, F., Merchionne, F. & Dammacco, F. (2002) Haematologica 87, 836-845. [PubMed] [Google Scholar]
- 32.Hughes, P. E., Diaz-Gonzalez, F., Leong, L., Wu, C., McDonald, J. A., Shattil, S. J. & Ginsberg, M. H. (1996) J. Biol. Chem. 271, 6571-6574. [DOI] [PubMed] [Google Scholar]
- 33.Wu, C., Hughes, P. E., Ginsberg, M. H. & McDonald, J. A. (1996) Cell Adhes. Commun. 4, 149-158. [DOI] [PubMed] [Google Scholar]
- 34.Seiki, M. (1999) Acta Pathol. Microbol. Immunol. Scand. 107, 137-143. [Google Scholar]
- 35.Ellerbroek, S. M. & Stack, M. S. (1999) BioEssays 21, 940-949. [DOI] [PubMed] [Google Scholar]
- 36.Brooks, P. C., Silletti, S., von Schalscha, T. L., Friedlander, M. & Cheresh, D. A. (1998) Cell 92, 391-400. [DOI] [PubMed] [Google Scholar]
- 37.Brooks, P. C., Stromblad, S., Sanders, L. C., von Schalscha, T. L., Aimes, R. T., Stetler-Stevenson, W. G., Quigley, J. P. & Cheresh, D. A. (1996) Cell 85, 683-693. [DOI] [PubMed] [Google Scholar]
- 38.Silletti, S., Kessler, T., Goldberg, J., Boger, D. L. & Cheresh, D. A. (2001) Proc. Natl. Acad. Sci. USA 98, 119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson, M. W., Toth, M., Gervasi, D. C., Sado, Y., Ninomiya, Y. & Fridman, R. (1998) J. Biol. Chem. 273, 10672-10681. [DOI] [PubMed] [Google Scholar]
- 40.Yu, Q. & Stamenkovic, I. (1999) Genes Dev. 13, 35-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huhtala, P., Tuuttila, A., Chow, L. T., Lohi, J., Keski-Oja, J. & Tryggvason, K. (1991) J. Biol. Chem. 266, 16485-16490. [PubMed] [Google Scholar]
- 42.Dong, J. T., Lamb, P. W., Rinker-Schaeffer, C. W., Vukanovic, J., Ichikawa, T., Isaacs, J. T. & Barrett, J. C. (1995) Science 268, 884-886. [DOI] [PubMed] [Google Scholar]
- 43.Seftor, R. E., Seftor, E. A., Koshikawa, N., Meltzer, P. S., Gardner, L. M., Bilban, M., Stetler-Stevenson, W. G., Quaranta, V. & Hendrix, M. J. (2001) Cancer Res. 61, 6322-6327. [PubMed] [Google Scholar]
- 44.Gross, T. J., Leavell, K. J. & Peterson, M. W. (1997) Thromb. Haemostasis 77, 894-900. [PubMed] [Google Scholar]
- 45.Deryugina, E. I., Ratnikov, B. I., Postnova, T. I., Rozanov, D. V. & Strongin, A. Y. (2002) J. Biol. Chem. 277, 9749-9756. [DOI] [PubMed] [Google Scholar]
- 46.Pampori, N., Hato, T., Stupack, D. G., Aidoudi, S., Cheresh, D. A., Nemerow, G. R. & Shattil, S. J. (1999) J. Biol. Chem. 274, 21609-21616. [DOI] [PubMed] [Google Scholar]