Abstract
Pulmonary artery (PA) hypertension was studied in a chronic hypoxic-pulmonary hypertension model (7–21 days) in the rat. Increase in PA pressure (measured by catheterism), cardiac right ventricle hypertrophy (determined by echocardiography), and PA remodeling (evaluated by histology) were almost entirely prevented after oral dehydroepiandrosterone (DHEA) administration (30 mg/kg every alternate day). Furthermore, in hypertensive rats, oral administration, or intravascular injection (into the jugular vein) of DHEA rapidly decreased PA hypertension. In PA smooth muscle cells, DHEA reduced the level of intracellular calcium (measured by microspectrofluorimetry). The effect of DHEA appears to involve a large conductance Ca2+-activated potassium channel (BKCa)-dependent stimulatory mechanism, at both function and expression levels (isometric contraction and Western blot), via a redox-dependent pathway. Voltage-gated potassium (Kv) channels also may be involved because the antagonist 4-amino-pyridine blocked part of the DHEA effect. The possible pathophysiological and therapeutic significance of the results is discussed.
Keywords: hypoxia, potassium channels
Exposure of animals to chronic hypoxia leads to the development of chronic hypoxic-pulmonary hypertension (CH-PHT). In human beings CH-PHT is frequently associated with severe pulmonary diseases. CH-PHT involve pulmonary arterial vasoconstriction and remodeling (1). Although the endothelium is involved in the pathogenesis of CH-PHT, the role of vascular smooth muscle cells (SMCs) is increasingly recognized (2).
Both the contractile status and the proliferative status of SMCs are regulated by the levels of intracellular Ca2+ ([Ca2+]i). The [Ca2+]i levels are determined in part by the influx of Ca2+ through the voltage-gated, L-type Ca2+ channels. In pulmonary artery (PA) SMCs, the membrane potential is regulated by large conductance Ca2+-activated channels (BKCa) (3) and voltage-gated K+ channels (Kv), including shaker family Kv (4, 5). K channel (BKCa and Kv) function and expression are down-regulated with development and maintenance of CH-PHT (6, 7). CH reduces K current density in PASMCs, resulting in a state of depolarization (8, 9), followed by elevation of [Ca2+]i, which induces contraction and proliferation (10).
The mechanism for K channel down-regulation is unclear, but recent work suggests that it is related to the altered redox state induced by CH (8). Lungs of rats with CH-PHT are in a more reduced redox state than those of normoxic controls, as indicated by increased levels of reduced glutathione (8). A reduced redox state has potential for both short-term effects through modulation of K+ channels function (11) and long-term effects by activating several oxygen-responsive genes including hypoxia-inducible factor (HIF) (12).
We sought to enhance expression and function of BKCa by using DHEA, a BKCa opener in hypoxic human pulmonary cells (13), which can shift the redox balance toward an oxidized state leading to both BKCa and Kv activation and thus repolarization of the PASMCs membrane potential and its effects on the [Ca2+]i. In vivo PA pressure (PAP) as well as right ventricular (RV) wall thickness were measured in closed-chest rats undergoing CH. Remodeling was determined in small and medium pulmonary arteries. In addition, the effect of DHEA on [Ca2+]i and the possible involvement of the potassium channels were measured by microspectrofluorimetry, isometric contraction measurement, and Western blot analysis.
Methods
Chronic Hypoxia and DHEA Treatments. Adult male Wistar rats (220–240 g) were randomized into five groups. Two groups were housed at normal atmospheric pressure (101 kPa); one group comprised rats treated with DHEA (30 mg·kg-1 orally every alternate day), which induces a circulating DHEA sulfate level of ≈0.2 μM after 3 wk (normoxic DHEA group) and another group did not receive DHEA (normoxic group). Other groups were kept in a hypobaric chamber (0.5 atm; 1 atm = 101.3 kPa) for 7–21 days: one group received DHEA (CH-DHEA group), another did not receive DHEA (CH group) and, in a third group, DHEA was given to the CH rats from day 15 to day 21 to look for CH-PHT regression. Intravascular administration was performed via the catheter inserted in the right jugular vein.
Hemodynamic and Echocardiography Measurements. Rats were anaesthetized with ketamine 50 mg/kg and xylazine 10 mg/kg by i.p. injection. Mean PAP was measured with 2.5-F catheters inserted into the right jugular vein in closed-chest rats (14). The effect of DHEA on the systemic blood pressure was controlled by another catheter placed into the left carotid artery. Throughout all the experiments, heart rate and oxygen saturation were monitored. Acute hypoxic stress with fraction of inspired O2 of 10% were applied by administration of an air plus nitrogen gas mixture. The fraction of inspired O2 was monitored with an oxygen analyzer (Servomex, Crowborough, Great Britain), and the effects on the PAP were measured over 10–15 min.
Echocardiography measurements were made in anaesthetized rats. After thorax epilation, the animals were placed in the left lateral decubitus position. Resting echocardiography measurements were performed at ambient conditions by using a SONOS 5500 (Philips) echocardiograph and a 12-MHz sector scare transducer. Right and left ventricle wall thickness was measured on M-mode tracings following the recommendations of the American Society of Echocardiology as described by Jones et al. (15). Right cardiac output was evaluated by measuring the electrical R-R delay, the PA diameter, and the velocity time integral (VTI) of PA flux. Of note, all the echocardiography measurements were measured by investigators blinded to the treatment groups.
Tissue and Cell Preparations. Heart and lungs were removed en bloc. Intrapulmonary arteries (IPAs) (150–300 μm of internal diameter) were then dissected under binocular control, and the adventicial and intimal layers were removed. For contraction experiments, rings (3 mm in length) were prepared. PASMCs were isolated by using an enzymatic protocol described (14).
Histology. A section of lung was formalin-fixed for histology studies. PA external diameter (PAED), PA internal diameter (PAID), and percentage vessel wall thickness (PAED - PAID)/PAED × 100) were measured in small- and medium-sized PAs (80–150 μm). Each group was comprised of four rats, and 10 measures were made per rat by an investigator blinded to the treatment groups.
[Ca2+]i Measurements. To assess the dynamic changes in [Ca2+]i of individual PASMC, we used the [Ca2+]i-sensitive fluorophore indo-1. PASMCs were loaded with indo-1 by incubation in physiological salt solution (139.6 mM NaCl/6.2 mM KCl/1 mM MgCl2/12.1 mM glucose/10 mM Hepes) containing 1 μM indo-1 penta-acetoxymethyl ester (Indo-1 a.m.) for 25 min at room temperature. The recording system included a Nikon Diaphot inverted microscope fitted with epifluorescence. The studied cell was illuminated at 360 nm and counted simultaneously at 405 nm and 480 nm by two photomultipliers (P100, Nikon). The fluorescence ratio (405:480) was calculated on-line and displayed with the two voltage signals on a monitor. [Ca2+]i was estimated from the 405:480 ratio (16).
Isometric Contraction Measurement. Isometric contractions were measured in rings from IPAs that were mounted in vertical 5-ml organ baths of a computerized isolated organ bath system (IOX, EMKA Technologies, Paris) as described (17). Baths were filled with Krebs–Henseleit solution (composition in mM: 118.4 NaCl/4.7 KCl/2.5 CaCl2/1.2 MgSO4/1.2 KH2PO4/25 NaHCO3/11.1 d-glucose, pH 7.4) maintained at 37°C and bubbled with a 95% O2/5% CO2 gas mixture. Tissues were set at optimal length by equilibration against a passive load of 10 and 20 mN for rings obtained from normoxic and hypoxic rats, respectively (15). A cumulative concentration-response curve was then constructed. A concentration increment was made once the maximal contractile effect of the preceding concentration had been recorded. Note that the endothelium was gently removed mechanically in all cases.
Western Blot Analysis. Arterial pulmonary extracts was prepared from a pool of eight rats per sample and condition. For each condition, three different samples were taken. All samples were ground in liquid N2, homogenized in RIPA buffer [1% Nonidet P-40/0.5% deoxycholate sodium/0.1% SDS/10 μg/ml aprotinin/100 μg/ml leupeptin/1 mM 4-(2-aminoethyl)benzenesulfonylfluoride in PBS] with a polytron (Bioblock Scientific), and then centrifuged. The extract was verified by Coomassie blue staining. Each sample was loaded at 10 μg in 7.5% resolving acrylamide gel and electrotransferred on to a nylon membrane. Immunoblot analyses used rabbit mAb against rat BKCa (Alomone Labs, Jerusalem, no. APC-021 at 1/500) and mouse monoclonal anti β-actin (Santa Cruz Biotechnology, clone C-2 at 1/500). The secondary Ab coupled with peroxidase was used at 1/5,000 dilution (Santa Cruz Biotechnology) and revealed with an enhanced chemiluminescence kit (Amersham Pharmacia). For quantification we used the scion image program from the National Institutes of Health. All experiments were repeated two or three times.
Chemicals and Drugs. Collagenase (type CLS1) was from Worthington. Pronase (type E), elastase (type 3), BSA, iberiotoxin (IbTx) (BKCa inhibitor), DHEA (prasterone), 1H-[1,2,4]oxadiazolol [4,3,-a]quinoxalin-1-one (ODQ) (GMPc pathway inhibitor), Genistein (tyrosine kinase inhibitor), “476485” a PKA inhibitor, 4-amino-pyridine (4-AP), DTT (a reducing agent), and agitoxin-2 (K shaker family blocker) were from Sigma. Indo-1 was from Calbiochem. DHEA, Indo-1, and (476485) were dissolved in DMSO or in ethanol. The maximal concentration of DMSO and ethanol used in our experiments was <0.1% and had no effect on the mechanical activity of rings and the resting value of the [Ca2+]i.
Analysis of Data. Values were expressed as mean ± SEM. Contractions were expressed as a percentage of K+-rich (80 mM) solution-induced contractions. Statistical analyses were performed by using NCSS 5.0 software (NCSS, Kaysville, UT). Intergroup differences were assessed by a repeated-measures ANOVA or factorial ANOVA, as appropriate. Post hoc analysis used a Fisher multiple comparison test. For some comparisons, the Pearson product moment correlation coefficient between pairs of variables was used as indicated (ORIGIN 5.0, OriginLab, Northampton, MA). Regarding the number of experiments, (n) refers to the number of rings or cells and (N) to the number of animals. Differences were considered significant when P < 0.05.
Results
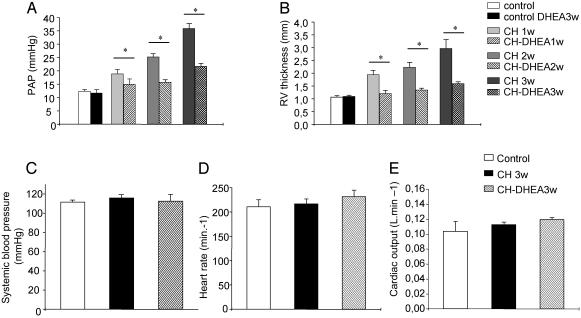
DHEA Prevents CH-PHT. DHEA administration to normoxic rats had no effect on both PAP and RV wall thickness (Fig. 1 A and B).
Fig. 1.
Effects of oral DHEA on pulmonary and systemic circulation. DHEA (3-wk oral) decreases the PAP (A) and RV wall thickness (B) but does not affect systemic circulation (C) of CH-DHEA rats or cardiac function (D and E).
Hypoxic exposure induced PAHT, as indicated by a significant increase in PAP from 12.5 ± 0.7 mmHg (N = 13) to 18.9 ± 1.7 (N = 4), 25.3 ± 1.14 (N = 5), and 34.5 ± 1.7 mm Hg (N = 13) in control, 1-, 2-, and 3-wk CH rats, respectively (P < 0.05) (Fig. 1 A). PAHT was also accompanied by an increase in the RV wall thickness from 0.107 ± 0.005 cm (N = 10) to 1.91 ± 0.16 mm (N = 5), 2.24 ± 0.2 mm (N = 7), and 2.74 ± 0.5 mm (N = 8) in control, 1-, 2-, and 3-wk CH rats, respectively (P < 0.05) (Fig. 1B). The variation in the RV wall thickness was significantly correlated to the increase in PAP (Pearson correlation coefficient = 0.84 P < 0.02) when determined after 7, 15, and 21 days of CH.
After 1 or 2 wk of hypoxia exposure with DHEA, no difference was observed in both PAP and RV wall thickness between CH-DHEA and control rats. After 3 wk of hypoxia exposure, the two parameters were significantly decreased in the CH-DHEA group compared with CH groups 21.7 ± 1.1 mm Hg (N = 9) vs. 34.5 ± 1.7 mm Hg (N = 13), and 1.6 ± 0.03 mm (N = 10) vs. 2.74 ± 0.5 mm (N = 8) (P < 0.05) (Fig. 1B).
No significant changes in systemic blood pressure, heart rate, and cardiac output were observed in the control, DHEA treated, and untreated groups (Fig. 1C).
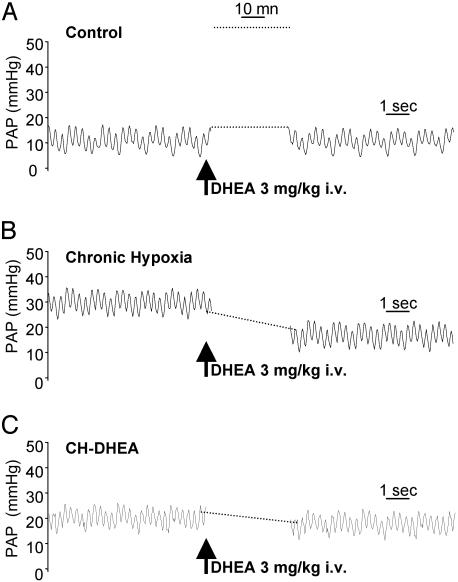
DHEA Partially Reverses Chronic PHT. Ten minutes after an acute intravascular treatment by DHEA (3 mg/kg) delivered within the PA through the catheter in 3-wk CH rats (Fig. 2), PAP significantly decreased from 34.5 ± 1.7 to 24.4 ± 2.5 mmHg (N = 6) (P < 0.05) (Fig. 2B). In 3-wk CH-DHEA rats, intravascular administration of DHEA did not further reduce PAP (Fig. 2C).
Fig. 2.
Effects of intravascular DHEA on mean PAP. Intravascular DHEA had no effect in both control and CH-DHEA-treated animals (A and C), whereas it induced a significant decrease in PAP in CH animals (B).
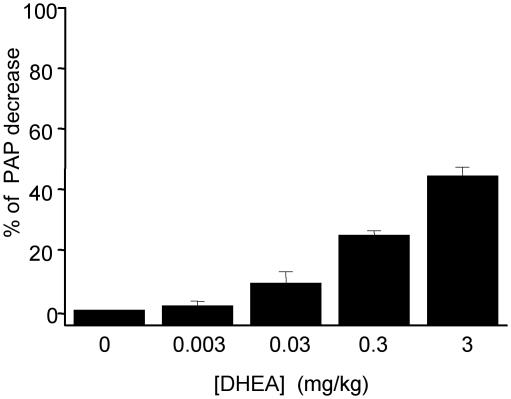
Remarkably intravascular administration of DHEA with successively doses from 30 μgto3mg/kg (N = 4) showed a decrease of the PAP in a dose-dependent manner (Fig. 3).
Fig. 3.
Effect of intravascular DHEA on mean PAP. Intravascular DHEA (3 μg to 3 mg/kg) decreased the PAP of CH rats in a dose-dependent manner.
Administration (during the last week) of oral DHEA in CH rats kept 3 wk in a CH environment, partially but significantly reversed both PAP and RV wall thickness: from 35.9 ± 1.9 mmHg (N = 9) to 21 ± 1.1 mm Hg (N = 3), and 2.97 ± 0.4 mm (N = 6) to 1.91 ± 0.2 (N = 4), P < 0.05, in treated (CH-DHEA 1 wk) and in untreated (CH) rats, respectively. As for the preventive treatments, DHEA treatments for 1 wk did not affect systemic pressure, cardiac output, and heart rate (data not shown).
DHEA Treatment Restores the Pressure Response of the PA to Acute Hypoxia. Normoxic rats responded to acute hypoxic challenge by an increase in PAP of 10.1 ± 0.4 mmHg (N = 5) after 10–15 min. After 3 wk of hypoxia, the pressure response to acute hypoxic challenge disappeared, as described in ref. 8. In contrast, under DHEA treatment, the response was restored (data not shown).
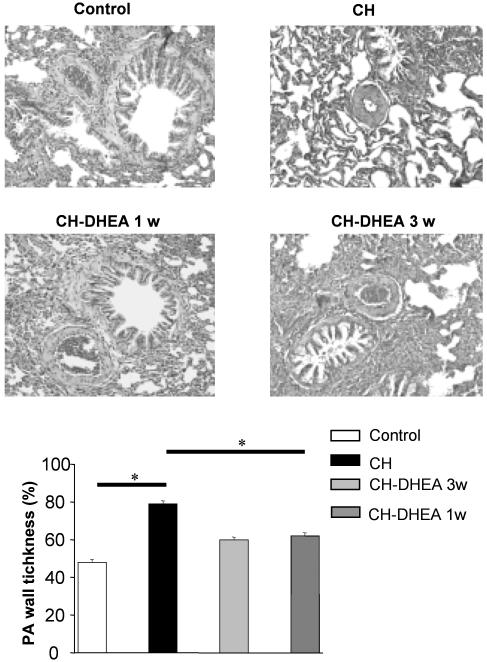
DHEA Treatment Prevents and Reduces CH-Induced IPA Remodeling. Three weeks of CH exposure induced a remodeling of the IPA (<150 μm) wall, which was characterized by an increase of 33% (n = 40; N = 4 each) in the media layer thickness compared with control rats (Fig. 4). In CH-DHEA rats, the media layer thickness was increased only 11% (n = 40; N = 4) compared with the control rats. The administration of DHEA during the last week, in rats kept 3 wk in a CH environment, partially but significantly reversed media wall thickness remodeling.
Fig. 4.
Effects of oral DHEA on PA remodeling. CH induced a significant increase in the PA wall thickness. Oral DHEA for 3 or 1 wk prevented and reversed PA wall remodeling, respectively.
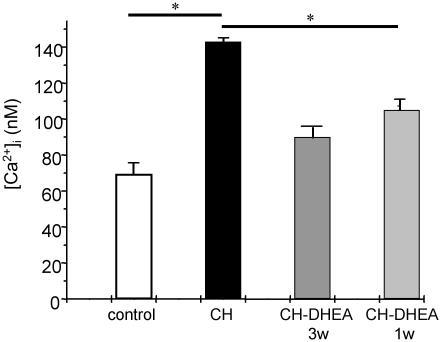
Effect of Chronic Treatment of Rats with DHEA on [Ca2+]i of PASMCs. CH induced a significant increase of the resting [Ca2+]i value of PASMCs from 69.2 ± 2.2 nM (n = 41; N = 4) to 143 ± 7 nM (n = 51; N = 4) in control and 3-wk CH rats, respectively (P < 0.05) (Fig. 5). This increase was significantly inhibited in PASMCs from CH-DHEA rats, in which the resting [Ca2+]i value was 90.1 ± 3.2 nM (n = 30; N = 4) (P < 0.05). When DHEA was administered from day 15 to day 21 of CH in another group of rats, the resting [Ca2+]i value was decreased significantly compared with the untreated rats: 105 ± 3 nM (n = 30; N = 4) vs. 143 ± 7 nM (n = 51; N = 4) (P < 0.05).
Fig. 5.
Effect of oral DHEA on [Ca2+]i of PASMCs. Oral DHEA for 3 or 1 wk induced a significant decrease in [Ca2+]i of PASMCs.
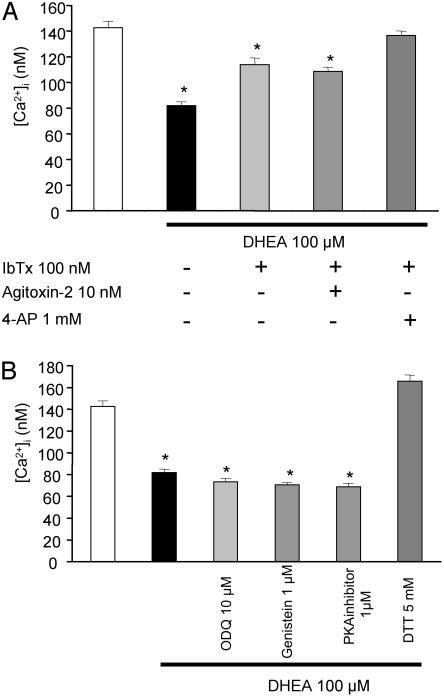
DHEA Activates BKCa and Kv Channels by a Redox-Sensitive Pathway. In vitro exposure for 15 min of PASMCs from CH rats to DHEA (100 μM) significantly decreased the resting [Ca2+]i value from 143 ± 7 nM (n = 51; N = 4) to 82 ± 3 nM, (n = 20; N = 4) (P < 0.05), (Fig. 6A). In contrast, DHEA (100 μM) did not alter [Ca2+]i in PASMCs from normoxic, normoxic-DHEA, and CH-DHEA rats (n = 30; N = 4 per group).
Fig. 6.
K channels and the mechanisms involved by in vitro DHEA administration on PASMCs. (A) DHEA (100 μM) induced a significant decrease in [Ca2+]i. IbTx (100 nM) blocked 65% of the DHEA-induced [Ca2+]i decrease, and combined IbTx and 4-AP (1 mM) was 100% efficient. Agitoxin-2 had no effect on the DHEA response. (B) DTT suppressed the DHEA-induced decrease in [Ca2+]i. The 1H-[1,2,4]oxadiazolol [4,3,-a]quinoxalin-1-one (ODQ), genistein, and a PKA inhibitor had no effect on DHEA-induced [Ca2+]i.
Treatment of PASMCs from CH rats with both DHEA (100 μM) and IbTx (100 nM) for 10 min partially inhibited the change induced by DHEA alone on the resting [Ca2+]i value by 62% (n = 30; N = 4) (Fig. 6A). The addition of agitoxin-2 had no effect (n = 15; N = 4). Combined presence of 4-AP and IbTx almost totally abolished the DHEA effect on the resting [Ca2+]i (n = 20; N = 4).
Several inhibitors of different signaling pathways were tested. Treatment of PASMCs with DHEA and 1H-[1,2,4]oxadiazolol [4,3,-a]quinoxalin-1-one (ODQ; 10 μM), or genistein (1 μM) or the protein kinase A inhibitor 476485 (1 μM) (n = 20; N = 4 each) did not alter the effect of DHEA on the resting [Ca2+]i value (Fig. 6B). In contrast, DTT, a reducing agent totally inhibited the effect of DHEA (n = 30; N = 4) and induced a slight elevation of the resting [Ca2+]i of 23 nM (Fig. 6B).
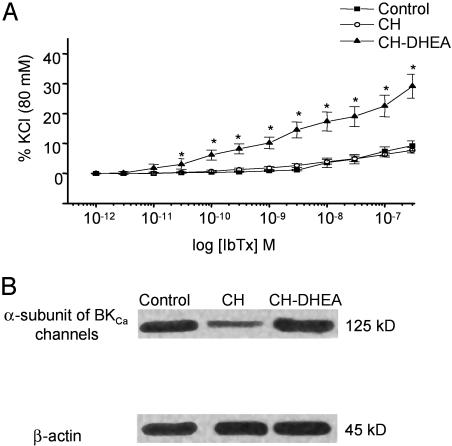
DHEA Increases BKCa Channel Activity and Expression in PA of CH Rats. CH had no significant effects on the IbTx induced contraction of IPA rings from CH rats (n = 11; N = 4) compared with control rats (n = 11; N = 4) (P > 0.05). DHEA increased the sensitivity for IbTx in IPA rings (Fig. 7A). The tension induced by 100 and 300 nM IbTx was increased by 52% and 55% in rings from CH-DHEA rats compared with control and CH rats.
Fig. 7.
Effects of DHEA on PA reactivity to BKCa blockers and BKCa expression. (A) In vitro concentration–response curves for the effect of IbTx on the resting tension of IPA rings from CH and CH-DHEA rats. The amplitude of contraction is expressed as a percentage of the KCl (80 mM)-induced response obtained at the beginning of the experiments. Note the increase in the IbTx response in rings from CH-DHEA rats. Data points are mean ± SEM for CH (n = 11, N = 4) and CH-DHEA (n = 11, N = 4) rats. (B) Immunoblots of the BKCa α-subunit after 21 day of oral administration of DHEA. Each lane was loaded with 10 μg of protein. BKCa α-subunit was recognized by the Ab as a 125-kDa immunoreactive band. BKCa α-subunit is down-regulated in CH vs. control groups, whereas its expression was similar between control and CH-DHEA groups. No difference was observed in the 45-kDa β-actin bands used as an internal standard.
The expression level of BKCa was studied in Western blot. Fig. 7B indicates a clear effect of CH, decreasing the expression of the BKCa α-subunit. This decrease was prevented by DHEA orally administrated for 3 wk; in three separate comparisons, the immunoreactive signal associated with the BKCa α-subunit was 48.6 ± 5.6% higher in CH-DHEA than CH.
Discussion
The present work conducted in a CH-PHT model indicates that DHEA prevents and reverses CH-PHT in rats and restores their normal response to an additional acute hypoxia. Increases in the PAP and RV hypertrophy are almost entirely prevented by DHEA treatment (30 mg/kg every 2 days). Furthermore, a single intravascular dose of DHEA or a 1-wk orally administered DHEA regimen in hypertensive rats significantly decreases PAP. We suggest that the effects of DHEA treatment on the function and expression of BKCa are the main factor explaining the ability of the compound to prevent and reverse CH-PHT and that the DHEA effects involve a redox-dependent pathway.
Human PHT is defined by a resting PAP >20 mmHg (18). PHT secondary to disorders of the respiratory system is the most frequent cause of PHT. Chronic hypoxia is the principal pathophysiological mechanism of this PHT. Two main drugs are actually used in treatment of PHT, inhaled nitric oxide and i.v. or s.c. enoprostenol (prostacyclin) that has been validated in primary PHT (19).
We developed hypoxia in rats by using a hypobaric chamber at 0.5 atm that corresponds to a stable hypoxia with an inspired fraction of oxygen of 10%. This model induces PHT similar to human PHT secondary to disorders of respiratory system, such as chronic obstructive pulmonary disease, interstitial lung disease, or neonatal chronic obstructive lung disease secondary of prematurity.
Very little DHEA is synthesized in rat adrenals and gonads, and the free plus conjugated DHEA concentration in the plasma is of the order of 1 nM. The active doses of DHEA used in our experiments, for chronic oral as well as acute intravascular administrations, induce circulating concentration of the order of ≥10-7 M of DHEA, as also are DHEA concentrations used in in vitro studies, and therefore our results are of a pharmacological nature. In rats, as well as in all animals, DHEA is metabolized to form a number of compounds including testosterone, estradiol, and Δ5-androstene-3β,17β-diol, to mention only those currently known as the most biologically important. The i.v. administration of testosterone, estradiol, and pregnenolone, following the same intravascular protocol in CH-DHEA rats for each compound (3 mg/kg) as for DHEA, did not reveal any effect of these steroids. In contrast, Δ5-androsten-3β,17β-diol,3βhydroxy-5α-androstan-17-one, and 3βmethyl-5α-androstan-17-one did reverse PA hypertension. Whether the steroids directly interact with KCa and/or Kv has not yet been demonstrated at the molecular level. In human beings, the circulating concentration of DHEA is of the order of 10-6 to 10-5 M, and the very majoritarian physiological sulfate form may have the potential to create efficacious levels of DHEA with reference to our rat experiments and effects recorded on chronically hypoxic human pulmonary smooth-muscle levels (13). Whether or not administration to human beings, orally or intravenously, may therapeutically improve dysfunction of PAMSC remains to be tested after in vitro studies confirming the DHEA sensitivity of human PAMSC [large DHEA amounts may be safely administered (20)].
Exposure of rats to hypoxia for 3 wk induced a significant 3-fold increase in the mean pulmonary blood pressure accompanied by RV hypertrophy and pulmonary vasculature remodeling. Our data clearly show that DHEA prevents or reverses CH-PHT development, RV hypertrophy, and PA remodeling and restores the PA contraction response to acute hypoxia. Furthermore, DHEA does not affect systemic pressure, cardiac outflow, left ventricle contraction, and heart rate, suggesting a specific activity of DHEA on pulmonary circulation.
At the cellular level, data clearly indicate that PAHT is associated with an increase in [Ca2+]i secondary to membrane depolarization of the PA myocytes (21). This leads to a deep change in the PA properties, and PA from CH rats do not respond to further acute hypoxic stress. DHEA has been characterized by Peng et al. (13) as a BKCa channel opener at 100 μM. IPA rings from CH-DHEA animal develop higher reactivity to IbTx, which blocks KCa as compared with CH rats, suggesting an involvement of the BKCa in the control of IPA basal tone from CH-DHEA rats. Furthermore, in smooth muscle cells from CH-PHT rats, acute exposure to DHEA induced a decrease of the resting [Ca2+]i that is blocked by IbTx. Western blot analysis indicates an up-regulation of the α-subunit BKCa in CH-DHEA as compared with CH rats.
Involvement of Kv is also possible because 4-AP partly suppresses the effect of DHEA on [Ca2+]i. However, the fact that agitoxine-2, a blocker of a subset of the Kv shaker family (Kv1.1, Kv1.3, and Kv1.6), has no effect suggests that these channels are not involved.
Under hypoxic conditions, the redox balance of the PA cells is in a reduced state, as described (8, 22). This reduced state is responsible in part for the K channel inhibition leading to CH-PHT. It has been suggested that DHEA may induce a decrease in the NADH:NAD ratio leading to oxidation of the cells (23). In view of this data, we hypothesize that a possible cellular mechanism, by which DHEA activates BKCa, may be correlated to a cellular oxidation leading to K channel activation. The fact that the reducing agent DTT (24, 25) inhibits the effect of DHEA on [Ca2+]i suggests that DHEA activated both BKCa and Kv by changing the redox balance toward a more oxidative state (23, 26). Nevertheless this hypothesis requires the precise determination of the redox balance in IPA extracts, which is very difficult because of the low quantity of tissue obtainable from IPA. Change in redox statute could also explain the increase in the BKCa expression as the oxidation of the cells is known to block the effect of hypoxia on HIF-1 (27).
DHEA is a very attractive drug for study in human PHT particularly because it has already been used in short- and long-term human studies without major toxicity (28). Furthermore, this DHEA activity on the vascular system seems to be specific for the pulmonary circulation. Thus DHEA may be beneficial in the treatment of pulmonary vascular diseases. Our results are in accordance with preliminary data of Hampl et al. (29), which became available to us after completion of this manuscript and suggest that chronic DHEA sulfate administration prevents chronic hypoxic PHT (CH-PHT).
Acknowledgments
We thank Professor Michel Lazdunski (Nice) and Professor Emmanuel Weitzenblum (Strasbourg) for commenting on the manuscript. We also thank Anne-Marie Lomenech, Pierre Techoueyre (Bordeaux), and Bernard Eychenne (Bicêtre) for technical assistance and Dominique Diop for editorial help. This study has been supported in part by the Centre de Recherche de l'Hôpital des Enfants de Bordeaux (CEDRE) laboratory of the Pediatric Hospital of Bordeaux and a grant of Artemis to the Baulieu-Fondation Nationale de Gérontologie (FNG) program.
Abbreviations: DHEA, dehydroepiandrosterone; Kv, voltage-gated K+ channels; HT, hypertension; PA, pulmonary artery; BKCa, large conductance Ca2+-activated channels; [Ca2+]i, intracellular Ca2+; CH-PHT, chronic hypoxic-pulmonary HT; IPA, intrapulmonary artery; PAP, PA pressure; RV, right ventricular; 4-AP, 4-amino-pyridine; PASMC, PA small muscle cell; IbTx, iberiotoxin.
References
- 1.Vender, R. L. (1994) Chest 106, 236-243. [DOI] [PubMed] [Google Scholar]
- 2.Michelakis, E. D. & Weir, E. K. (2001) Clin. Chest Med. 22, 419-432. [DOI] [PubMed] [Google Scholar]
- 3.Peng, W., Hoidal, J. R., Karwande, S. V. & Farrukh, I. S. (1997) Am. J. Physiol. 272, C1271-C1278. [DOI] [PubMed] [Google Scholar]
- 4.Archer, S. L., Weir, E. K., Reeve, H. L. & Michelakis, E. (2000) Adv. Exp. Med. Biol. 475, 219-240. [DOI] [PubMed] [Google Scholar]
- 5.Patel, A. J., Lazdunski, M. & Honore, E. (1997) EMBO J. 16, 6615-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platoshyn, O., Yu, Y., Golovina, V. A., McDaniel, S. S., Krick, S., Li, L., Wang, J. Y., Rubin, L. J. & Yuan, J. X. (2001) Am. J. Physiol. 280, L801-L812. [DOI] [PubMed] [Google Scholar]
- 7.Olschewski, A., Hong, Z., Linden, B. C., Porter, V. A., Weir, E. K. & Cornfield, D. N. (2002) Am. J. Physiol. 283, L1103-L1109. [DOI] [PubMed] [Google Scholar]
- 8.Reeve, H. L., Michelakis, E., Nelson, D. P., Weir, E. K. & Archer, S. L. (2001) J. Appl. Physiol. 90, 2249-2256. [DOI] [PubMed] [Google Scholar]
- 9.Smirnov, S. V., Robertson, T. P., Ward, J. P. & Aaronson, P. I. (1994) Am. J. Physiol. 266, H365-H370. [DOI] [PubMed] [Google Scholar]
- 10.Platoshyn, O., Golovina, V. A., Bailey, C. L., Limsuwan, A., Krick, S., Juhaszova, M., Seiden, J. E., Rubin, L. J. & Yuan, J. X. (2000) Am. J. Physiol. 279, C1540-C1549. [DOI] [PubMed] [Google Scholar]
- 11.Reeve, H. L., Weir, E. K., Nelson, D. P., Peterson, D. A. & Archer, S. L. (1995) Exp. Physiol. 80, 825-834. [DOI] [PubMed] [Google Scholar]
- 12.Huang, L. E., Arany, Z., Livingston, D. M. & Bunn, H. F. (1996) J. Biol. Chem. 271, 32253-32259. [DOI] [PubMed] [Google Scholar]
- 13.Peng, W., Hoidal, J. R. & Farrukh, I. S. (1999) Am. J. Respir. Cell Mol. Biol. 20, 737-745. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet, S., Dubuis, E., Vandier, C., Martin, S., Marthan, R. & Savineau, J. P. (2002) Cardiovasc. Res. 53, 1019-1028. [DOI] [PubMed] [Google Scholar]
- 15.Jones, J. E., Mendes, L., Rudd, M. A., Russo, G., Loscalzo, J. & Zhang, Y. Y. (2002) Am. J. Physiol. 283, H364-H371. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet, S., Belus, A., Hyvelin, J. M., Roux, E., Marthan, R. & Savineau, J. P. (2001) Am. J. Physiol. 281, L193-L201. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet, S., Hyvelin, J. M., Bonnet, P., Marthan, R. & Savineau, J. P. (2001) Am. J. Physiol. 281, L183-L192. [DOI] [PubMed] [Google Scholar]
- 18.Weitzenblum, E. (2003) Heart 89, 225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vachiery, J. L., Hill, N., Zwicke, D., Barst, R., Blackburn, S. & Naeije, R. (2002) Chest 121, 1561-1565. [DOI] [PubMed] [Google Scholar]
- 20.Tummala, S. & Svec, F. (1999) Clin. Biochem. 32, 355-361. [DOI] [PubMed] [Google Scholar]
- 21.Yuan, X. J. (1995) Circ. Res. 77, 370-378. [DOI] [PubMed] [Google Scholar]
- 22.Yuan, X. J., Tod, M. L., Rubin, L. J. & Blaustein, M. P. (1995) Exp. Physiol. 80, 803-813. [DOI] [PubMed] [Google Scholar]
- 23.Gupte, S. A., Li, K. X., Okada, T., Sato, K. & Oka, M. (2002) J. Pharmacol. Exp. Ther. 301, 299-305. [DOI] [PubMed] [Google Scholar]
- 24.Park, M. K., Lee, S. H., Ho, W. K. & Earm, Y. E. (1995) Exp. Physiol. 80, 835-842. [DOI] [PubMed] [Google Scholar]
- 25.Park, M. K., Bae, Y. M., Lee, S. H., Ho, W. K. & Earm, Y. E. (1997) Pflügers Arch. 434, 764-771. [DOI] [PubMed] [Google Scholar]
- 26.Swierczynski, J., Slominska, E., Smolenski, R. T. & Mayer, D. (2001) Pol. J. Pharmacol. 53, 125-130. [PubMed] [Google Scholar]
- 27.Sylvester, J. T. (2001) Circ. Res. 88, 1228-1230. [DOI] [PubMed] [Google Scholar]
- 28.Baulieu, E. E., Thomas, G., Legrain, S., Lahlou, N., Roger, M., Debuire, B., Faucounau, V., Girard, L., Hervy, M. P., Latour, F., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 4279-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampl, V., Bibova, J., Povysilova, V. & Herget, J. (2003) Eur. Respir. J. 21, 862-865. [DOI] [PubMed] [Google Scholar]