Abstract
Early detection of colorectal cancer is critical for the management of this disease. Biomarkers for early detection of several cancers have been developed and applied clinically in recent years. We have sought to discover candidate biomarkers without the restricted choice of markers placed on microarrays, and without the biological complications of genetic and environmental heterogeneity. We have compared by cDNA subtraction two genetically matched sets of mice, one developing multiple intestinal neoplasia (C57BL/6J-ApcMin) and the other tumor-free (C57BL/6J). One prominent candidate biomarker, clusterin, was then subjected to a series of validation steps. In situ hybridization and immunohistochemistry were used to analyze clusterin expression at a cellular level on a series of murine intestinal and human colonic neoplasms. Elevated clusterin expression was characterized within certain regions of murine and human tumors regardless of tumor stage, location, or mode of initiation. The cells showing high clusterin levels generally lacked differentiation markers and adenomatous polyposis coli antigen. Tumor cells undergoing apoptosis expressed low levels of clusterin. Its specific expression patterns and correlation with cellular events during tumorigenesis make it a useful diagnostic tool in the mouse and a potential contributor to the set of biomarkers for early detection of human colon cancer.
Colorectal cancer ranks third among all cancers in terms of occurrence and death rate among both male and female populations of the United States (1). Yet, like many other cancers, colorectal cancer can be completely cured by surgery if detected at early stages (2). Current methods for the early detection of this cancer include the fecal occult blood test, flexible sigmoidoscopy, double-contrast barium enema, and colonoscopy (1). However, each of these suffers from inaccuracy and/or poor compliance; therefore, it would be valuable to develop new methods to effectively detect early colorectal tumors.
Changes in gene expression profile occur as tumors develop and progress (3). Tumor suppressor genes are commonly suppressed, while oncogenes are activated in this process. In addition, the expression levels of many other related genes are also altered, owing to the dramatic functional changes of the cell population and/or regulation within tumors. Study of the expression patterns of tumor-related genes provides not only valuable information for the biology of cancer but also indicators for cancer detection and diagnosis. In recent years, tumor-associated molecules, termed biomarkers, have been identified and developed as sensitive indicators of certain early tumors. For example, elevated prostate-specific antigen is associated with prostate cancer (4); nuclear matrix protein, fibrin/fibrinogen product, and bladder tumor antigen are potential biomarkers for bladder cancer (5); estrogen receptors, mutant p53, bcl-2, and HER-2 neu oncogenes are currently the most useful markers for the detection of breast cancer (6); and α-fetoprotein serves as an accurate monitor during progression and chemotherapy of certain forms of testicular cancer (7). The use of molecular biomarkers offers both convenience and accuracy compared with other detection methods.
Seeking molecular biomarkers for intestinal tumors, we compared RNA of intestinal tissues collected from WT C57BL/6J (B6) and from C57BL/6J-ApcMin/+ (B6-Min) mice. The latter strain, carrying the multiple intestinal neoplasia (Min) mutation in the adenomatous polyposis coli (Apc) gene (8), develops tumors throughout the intestinal tract (9). This line provides an experimental model for one type of the human familial intestinal cancers (10). With PCR-based subtractive hybridization and differential screening, genes with elevated expression in the tumor-bearing intestine were identified.
One of the molecules identified by this procedure is clusterin. Clusterin mRNA and protein were both elevated in intestinal tumors. Because the cellular functions of clusterin are not yet known, we further investigated the relationships between clusterin expression and loss of Apc function, intestinal cell differentiation, cell proliferation, and apoptosis. Further, we have established that elevated clusterin expression is found in both early and late intestinal neoplasms in the mouse, regardless of position in the intestinal tract or mode of tumor initiation. Finally, we have found that expression of the clusterin gene is altered in human colorectal cancer: clusterin antigen is elevated in early intestinal lesions, benign polyps, adenocarcinomas, and normal epithelia adjacent to tumors.
Materials and Methods
Mouse Husbandry and Genotyping. The B6 WT, B6-Min, and SWR/J (SWR) mice were obtained from The Jackson Laboratory. All mice were bred, housed, and genotyped for Min in the American Association of Laboratory Animal Care-approved animal facility of the McArdle Laboratory as described (8, 11).
RNA and mRNA Isolation. The entire intestinal tracts from WT and Min mice were removed at 100 days of age and fixed immediately in RNAlater RNA Stabilization Reagent (10 ml/g of wet weight; Qiagen, Valencia, CA). Total RNA and mRNA samples were isolated with TRIzol reagent (Invitrogen) and a Poly(A) Purist mRNA Isolation Kit (Ambion, Austin, TX) respectively, according to the manufacturers' protocols.
Suppression Subtractive Hybridization and Differential Screening. Subtractive hybridization with mRNA samples from tumor-bearing intestines and normal intestines and subsequent differential screening were performed with the PCR-Select cDNA Subtraction Kit and the PCR-Select Differential Screening Kit (CLONTECH), according to the manufacturer's protocols. The resulting clones were sequenced on an Applied Biosystems 377 sequencer, and their sequences were compared with those in GenBank by using the BLAST search tool.
Nonradioactive in Situ Hybridization. RNase-free paraffin-embedded sections of intestinal tumors were prepared as described. The protocol for nonradioactive in situ hybridization (12), generously communicated by Chris Iacobuzio-Donahue (The Johns Hopkins University, Baltimore), was followed with modification. The complete modified protocol can be found in Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org.
Immunohistochemistry and Immunohistofluorescence Assay. Immunohistochemistry and immunofluorescence assays were performed with paraffin sections of mouse and human tumors as described (13) with minor modifications. The protocols can be found in Supporting Materials and Methods.
Fluorescent Terminal Deoxynucleotidyltransferase-Mediated dUTP-Biotin Nick End Labeling (TUNEL) Assay. The ApopTag Apoptosis Detection Kit (Intergen, Purchase, NY) was used on paraffin sections, following the manufacturer's protocol.
Results
Subtractive Hybridization and Differential Screening. PCR-based subtractive hybridization was performed by using cDNA templated by RNA from the entire intestines of tumor-bearing Min mice from which cDNA from WT mice had been subtracted, all on the B6 background. The amplified cDNA fragments were then subcloned. After transformation, 1,056 white colonies were randomly selected and cultured. The subsequent differential hybridization screening revealed 127 clones with stronger signals with cDNA probes templated by RNA from tumor-bearing intestines than with cDNA probes from normal intestines. Sequencing revealed 71 distinct clones, including 12 not previously described. Twenty different clones (three novel) were used as probes for in situ hybridization; eight (one novel) demonstrated elevated expression in tumor cells. Interestingly, 1 of the 8 confirmed positive clones encoded clusterin, a widely distributed glycoprotein with undefined functions (14), which is also represented by 6 of 127 of the original sequenced clones. Because clusterin was previously reported to be associated with apoptosis and several human cancers (14), it was of great interest to study, in depth, the expression of clusterin in intestinal tumorigenesis.
Expression Pattern of Clusterin in Min Tumors. As a preliminary experiment, a Northern blot analysis with a clusterin cDNA probe confirmed the highly elevated level of clusterin in tumor-bearing intestines compared with normal (data not shown).
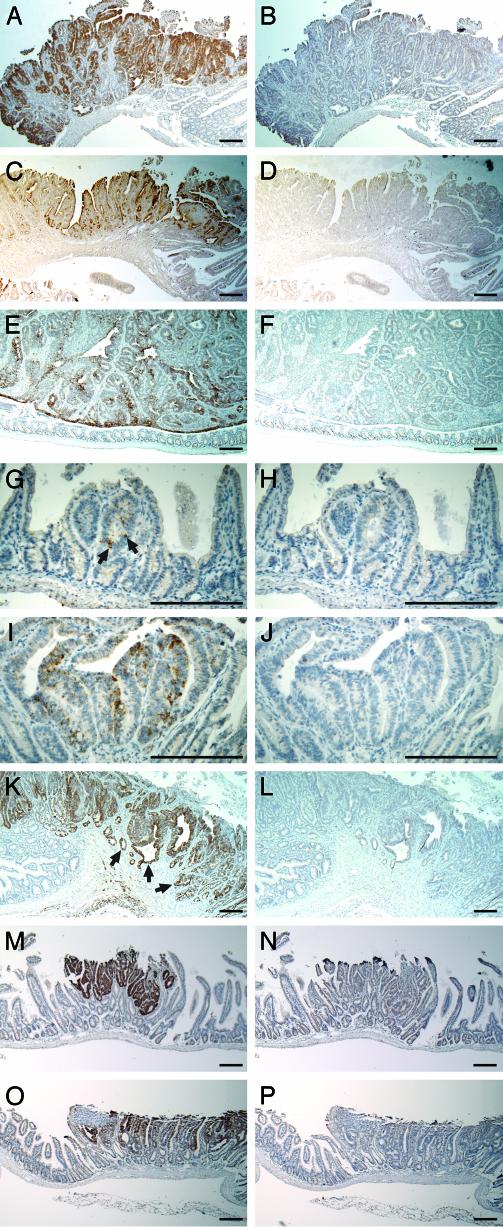
The fine histological preservation in paraffin sections of tissues fixed with formalin enables us to investigate more deeply the cellular significance of the differential signal. Nonradioactive in situ hybridization was then used to characterize the spatial expression of clusterin. The path toward a deeper understanding starts with an analysis of expression at cellular resolution. Throughout the small intestinal tract, strong clusterin signals were localized only in the tumors (Fig. 1 A and B), and these signals were not detectable in the adjacent normal epithelium or in the muscularis or lamina propria. This result was confirmed by immunohistochemistry (Fig. 1 C and D). Staining for the clusterin antigen in the tumors was much stronger than that in normal tissues. The majority of the antigen was localized at the edges of tumor tissue toward the luminal cavities, suggesting that clusterin is secreted. Moderately to highly elevated levels of clusterin RNA were also detected in colonic tumors (Fig. 1 E and F).
Fig. 1.
Expression of clusterin in murine intestinal tumors. (A, E, G, I, K, M, and O) The staining (brown) of clusterin RNA with antisense probe by in situ hybridization. (C) The staining of clusterin protein with clusterin antibody by immunohistochemistry. (B, F, H, J, L, N, and P) Negative controls with sense probe. (D) Negative control lacking the primary antibody. Highly elevated clusterin RNA signal (A and B) and increased production of clusterin antigen (C and D) were detected in the tumors of small intestines. Elevated clusterin RNA signal was shown in colon tumors (E and F). Noticeable elevation of the clusterin signal is indicated by arrows (G) in the microadenomas from 29-day-old Min mice (G and H). Gradual elevation of clusterin RNA was observed in early adenomas from 29-day-old Min mice (I and J). Invasive adenocarcinomas in 270-day-old mouse (SWR × B6-Min)F1 demonstrated highly elevated levels of clusterin (K and L). The arrows in K point to invasive tumor tissue. Up-regulation of clusterin RNA was also observed in N-ethyl-N-nitrosourea-induced adenomas (M and N) and in tumors arising from allelic silencing of Apc+ (O and P) in the small intestine. (Bars: 200 μm.)
To study the temporal expression pattern of clusterin, we collected and analyzed tumors at early and late stages of tumorigenesis. Microadenomas, normally involving no more than five crypts, as well as small adenomas up to 0.5 mm in diameter, were isolated from the small intestines of 29-day-old Min mice. Weak but noticeable up-regulation of the clusterin RNA signal was detected in some microadenomas by in situ hybridization (Fig. 1 G and H, indicated by arrows). With further development of the tumor, clusterin RNA levels seemed to be gradually elevated in small adenomas (Fig. 1 I and J). Invasive adenocarcinomas were isolated from an (SWR × B6-Min)F1 mouse at the age of 270 days. Cells in these tumors, including those invading the muscular layers, demonstrated strong expression of clusterin RNA (Fig. 1 K and L). Thus, the level of clusterin RNA increases as tumors progress.
Clusterin Expression in N-ethyl-N-nitrosourea-Induced Tumors and in Tumors Maintaining Apc Heterozygosity. We examined tumors induced by a strong mutagen, N-ethyl-N-nitrosourea, and tumors initiated by silencing the WT Apc allele (maintenance of heterozygosity) (15) for clusterin expression by in situ hybridization. Both tumor types (Fig. 1 M–P) demonstrated elevated clusterin expression similar to B6-Min tumors, which are normally initiated through losing the WT Apc allele by somatic recombination [loss of heterozygosity (LOH)] (16, 17). These results indicated that the elevated clusterin expression in intestinal tumors is not restricted to Min-induced or LOH tumors.
Clusterin Expression and Cellular Events in Tumors. The pattern of clusterin expression was heterogeneous in tumors (Fig. 1 A, E, and K), perhaps reflecting different features of these cells. Such potential features were then explored with cellular markers, as described below.
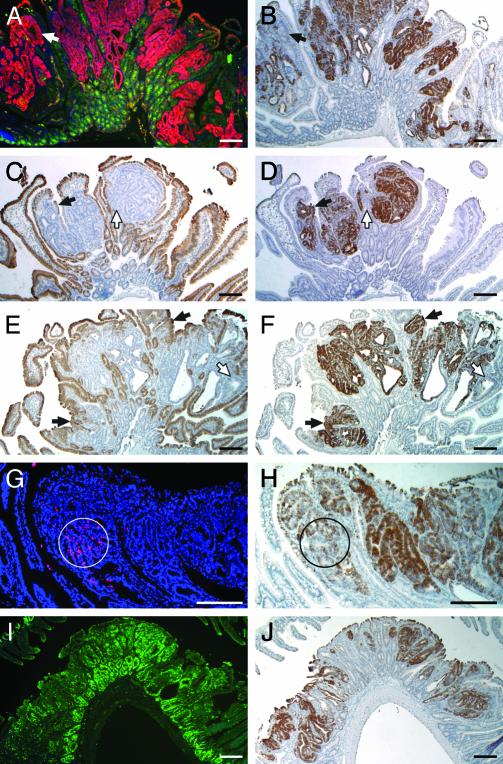
Lack of Apc function, by loss, mutation, or silencing of the Apc WT allele, is a frequent event in intestinal tumorigenesis (18, 19). Because the Apc protein is involved in degradation of β-catenin within the cytoplasm, loss of WT Apc leads to enhanced levels of cytoplasmic β-catenin protein (20, 21). Tumor cells were therefore identified by dual-staining immunohistochemistry for Apc and β-catenin antigens (Fig. 2A). In situ hybridization for clusterin on an adjacent section (Fig. 2B) indicated that these tumor cells commonly expressed a high level of clusterin. Clusterin-negative exceptions are indicated by arrows in Fig. 2 A and B.
Fig. 2.
Analysis of clusterin expression patterns. (A) Dual-staining immunofluorescence assay on a Min tumor section demonstrated that the WT Apc antigen (green) was present in the normal crypts, but not in tumor cells, where a high level of β-catenin antigen (red) was detected. (B) Staining of clusterin RNA (dark brown) with antisense probe in an adjacent section is similar to that of cytoplasmic β-catenin protein. The arrows in A and B point to a small exceptional region lacking both WT Apc antigen and clusterin antigen. (C–F) Expression of differentiation markers and clusterin RNA in intestinal tumors. (A, C, E, G, and I) RNA signals (dark brown) of two differentiation markers, intestinal alkaline phosphatase (C) and small proline-rich protein 2a (E), with their antisense probes. (B, D, F, H, and J) Sections adjacent to C and E were hybridized for clusterin RNA (dark brown) with the antisense probe for comparison (D and F). Solid arrows in C–F point to small exceptional regions expressing one of the differentiation markers and clusterin, whereas open arrows point to small regions lacking both of them. (G) Cells undergoing apoptosis within tumors were labeled red by fluorescent TUNEL assay, in which the nuclei were stained blue by 4′,6-diamidino-2-phenylindole (DAPI). (H) For comparison, a section adjacent to G was hybridized for clusterin RNA (dark brown) with the antisense probe. Circles indicate the regions showing a high apoptotic index, where clusterin RNA levels were relatively low. (I) A section of tumor stained for Ki67 antigen by immunofluorescence (green signal). (J) A section adjacent to I hybridized for clusterin RNA (dark brown) with the antisense probe. (Bars: 200 μm.)
Actively proliferating tumor cells commonly lack terminal differentiation markers (22, 23). Some cells within intestinal adenomas do, however, express epithelial differentiation markers (24). For example, in the normal intestinal epithelium, two differentiation markers, intestinal alkaline phosphatase (iAP) and small proline-rich protein 2a (sprr2a), are found at high levels in villi, but not in crypts. Yet in intestinal tumors, we detected the expression of iAP or sprr2a RNAs in a few tumor cells (Fig. 2 C and E). These heterogeneous expression patterns of iAP and sprr2a led us to investigate a correlation between clusterin expression and the differentiation status of cells within tumors. In situ hybridization for clusterin RNA on adjacent sections revealed that the majority of cells expressing high levels of clusterin lacked both differentiation markers (Fig. 2 D and F). Conversely, the majority of cells expressing these markers lacked clusterin expression. This pattern of reciprocal expression between clusterin and markers for terminal differentiation was not universally observed, however. Some cells close to the edge of tumors expressed both clusterin and differentiation markers (indicated by solid arrows in Fig. 2 C–F), whereas other tumor cells lacking both clusterin and differentiation markers could also be found (indicated by open arrows in Fig. 2 C–F). Nevertheless, the canonical observation was that of reciprocal expression.
Clusterin and Apoptosis. Previous reports of different experimental models have presented both positive and negative correlations between clusterin expression and apoptotic index (14). The fluorescent TUNEL assay, which specifically stains cells undergoing apoptosis (25), was performed to investigate a correlation between apoptosis and elevation of clusterin expression within intestinal tumors. These assays generally showed a scattered distribution of cells undergoing apoptosis in most tumor sections (Fig. 2G), with some regions showing pockets of apoptotic cells (representative example circled in Fig. 2G). Within sections of 18 different tumors from the small intestines of Min mice, 27 regions showed a high apoptotic index (>10 TUNEL-positive cells within a circle 200 μm in diameter). In situ hybridization for clusterin RNA on adjacent sections indicated that 24 of these 27 regions, mostly in the basal regions or edges of the tumors, had relatively low levels of clusterin RNA (example circled in Fig. 2H). By contrast, most tumor cells showing high levels of clusterin RNA, generally located in the central and luminal regions of tumors, did not show elevated apoptotic indices. Therefore, in general, clusterin RNA levels are negatively correlated with groups of apoptotic cells in tumors.
Clusterin and Cell Proliferation. Because clusterin expression is negatively correlated with both terminal differentiation and apoptosis in the Min mouse model, the relationship between clusterin levels and cell proliferation was investigated (23). A rabbit polyclonal antibody against Ki67 antigen (Vector Laboratories) was used for immunofluorescence assays on sections of Min intestinal tumors. As expected, the cells within the tumors and at the base of crypts demonstrated strong proliferation signals (Fig. 2I), and staining by Ki67 in tumors was heterogeneous. In situ hybridization for clusterin RNA on adjacent sections (Fig. 2 J) indicated that elevated clusterin expression in intestinal tumors was not correlated with Ki67 antigen distribution.
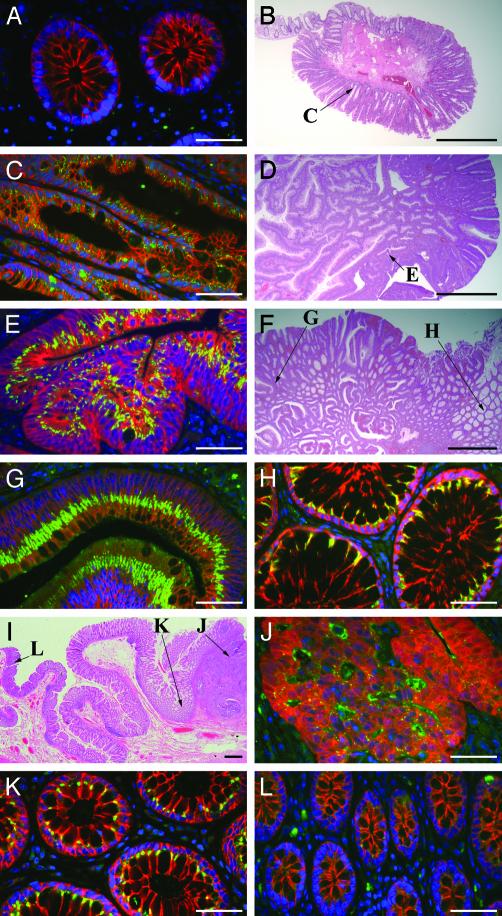
Clusterin Production in Human Colon Tumors. Anonymous and archived human colon tissue samples collected during routine colonoscopy were acquired with approval from the Human Subjects Committee of the Institutional Review Board at the University of Wisconsin. Immunofluorescence assays for clusterin were performed with a polyclonal antibody against human clusterin (Research Diagnostics, Flanders, NJ) on sections histopathologically diagnosed as normal colonic tissues (n = 2), hyperplastic polyps (n = 3), tubular adenomas (n = 1), villous adenomas (n = 1), or invasive adenocarcinomas (n = 1). Very low levels of clusterin antigen were detected in the cytoplasm of normal colonic epithelial cells (Fig. 3A). Hyperplastic polyps (Fig. 3B), commonly regarded as minor intestinal lesions and often found during colonoscopy, displayed weak clusterin levels in the crypts (Fig. 3C, located as shown in Fig. 3B). Tubular adenomas (Fig. 3D) have characteristic finger-like structures in sections and showed highly elevated clusterin antigen in the cytoplasm on the apical side, as well as multiple layers of nuclei and elevated cytoplasmic β-catenin (Fig. 3E, located as shown in Fig. 3D). The villous adenoma (Fig. 3F), another type of benign tumor collected in colonoscopy, displayed high levels of clusterin in the cytoplasm of the apical side in the tumor epithelial cells (Fig. 3G, indicated as G in Fig. 3F). Interestingly, the epithelial cells in the morphologically normal crypts close to the adenoma (within 5 mm) also showed a strong clusterin signal in the cytoplasm near the apical cell membrane (Fig. 3H, indicated as H in Fig. 3F). The most advanced colonic tumor investigated was an invasive adenocarcinoma (upper right area of Fig. 3I). At this tumor stage, immunohistochemistry demonstrated that within the tumor cells that lack basal–apical polarization, where cytoplasmic β-catenin antigen was highly elevated, the clusterin antigen level was relatively weak compared with that in adenomas. Clusterin antigen can be seen in the intercellular cavities (Fig. 3J, located at J of Fig. 3I). Remarkably, a strong clusterin signal was observed in the epithelial cells of normal crypts adjacent to the adenocarcinoma (Fig. 3K, located at K of Fig. 3I). By contrast, in the crypts >1 cm from the edge of the adenocarcinoma, the cytoplasmic clusterin signal was weak, similar to that in normal colonic epithelium (Fig. 3L, shown at L in Fig. 3I).
Fig. 3.
Localization of clusterin protein in various human colorectal tissues. Dual-staining immunofluorescence assays (A, C, E, G, H, J, K, and L) stained clusterin antigen (green-yellow), β-catenin (red), and nuclei (blue) in the human colorectal tissues. Hematoxylin/eosin stains of hyperplastic polyp (B), tubular adenoma (D), villous adenoma (F), and invasive adenocarcinoma (I) have arrows to indicate the positions of corresponding immunostaining panels. Normal colonic crypts (A) have minimal clusterin antigen and no β-catenin in the epithelial cytoplasm. Hyperplastic polyps (C) have noticeable elevation of clusterin protein, but not β-catenin, in the epithelial cytoplasm. Tubular adenomas (E) and villous adenomas (G) have highly elevated clusterin antigen in the apical side of epithelial cytoplasm, along with increased cytoplasmicβ-catenin. Normal crypts adjacent to adenomas (H) show strong clusterin signals in the apical epithelial cytoplasm. Invasive adenocarcinomas (J) with highly elevated cytoplasmic β-catenin have detectable clusterin antigen in the cytoplasm and strong clusterin signals in the intercellular cavities of the tumor. Normal crypts close to the adenocarcinoma (within 0.5 cm from the edge) (K) show fairly strong clusterin signals in the apical cytoplasm. However, normal crypts >1 cm from the edge of the adenocarcinoma (L) have only minimal clusterin antigen in the cytoplasm. (Bars: 50 μmin A, C, E, G, H, and J–L;1mm in B, D, F, and I.)
Discussion
The identification of useful markers for detection and diagnosis of human colon cancer is a major goal of our research. A basic strategic principle is to use the homogeneous Min mouse model to generate candidate markers and then to test them on the more genetically and environmentally heterogeneous human materials. We have used PCR-based subtractive hybridization (26) to detect tumor-associated genes by enriching the cDNA of genes with higher expression levels (27), either intrinsic to the tumor or indirectly elevated in the tumor-bearing tissue. False positives can be eliminated by Northern blotting and in situ hybridization. The latter ascertains the cellular region of differential expression. The subtraction approach is not stoichiometric, so that positive signals can be missed. However, it can detect novel cDNAs that may be absent from a microarray. Interestingly, clusterin, the marker gene described in this report, is absent from the list of 108 genes with elevated expression in colonic tumors in the serial analysis of gene expression (SAGE) database (28). Absence of clusterin probably reflects the different sources of comparison. We have compared tissues from a pair of isogenically matched mice. By contrast, the SAGE comparison was made between human primary colon tumors and cultured human colon cancer cells versus cultured normal colon cells.
Clusterin, also known as apolipoprotein J (29, 30), TRPM-2 (31, 32), or SGP-2 (33), is an enigmatic glycoprotein. Its expression is widely distributed in various tissues (14, 34). Although identified two decades ago, its functions have not yet been fully elucidated (14, 34). Previous reports have correlated clusterin expression with cell responses to stress (35), cell damage recovery (36, 37), senescence (38, 39), tumorigenesis (14, 40), and apoptosis (14). In this report, we have demonstrated that clusterin expression is relatively uniform despite the regional differences of intestinal tumors in average size, frequency, and morphology (9, 24). This uniformity suggests the importance of clusterin expression in general tumor physiology.
Up-regulation of clusterin in intestinal tumors has not been previously reported, although its high expression has been reported in several in vivo cancers and cancer cell lines (14). The antibody used for immunohistochemistry was produced by using a highly glycosylated, secreted form of clusterin protein. As shown above, this antibody successfully detects the clusterin secreted from the tumor cells. Nevertheless, nonglycosylated clusterin could also be present intracellularly but not detected in our immunohistochemistry assay (41). Thus, we prefer to judge clusterin expression on the basis of in situ hybridization.
Min tumors arising on different genetic backgrounds have allowed us to study the expression of clusterin at all tumor stages (42) from microadenomas through invasive adenocarcinomas (43), but not yet metastatic carcinomas. Expression patterns of clusterin at different stages indicate that this protein functions in the maintenance and/or progression of tumors, but it is not clear whether it plays a role in tumor establishment. Note that elevated clusterin expression is qualitatively maintained in both murine and human invasive adenocarcinomas.
We have investigated correlations with other cellular parameters within fields of cells that are heterogeneous in clusterin expression. This heterogeneity in intestinal tumors reflects the cellular architecture involving multiple cell types and allowed us to investigate correlations between clusterin expression and cellular events that are critical to tumorigenesis.
Loss of tumor suppressor Apc function initiates tumorigenesis in the intestine (44, 45). Cells expressing normal Apc protein within the tumors appear to be normal, except for hyperplasticity. Our results indicate a strong positive association between elevation of clusterin expression and loss of Apc function in tumor cells. This correlation was further confirmed by our results with N-ethyl-N-nitrosourea-induced and maintenance of heterozygosity tumors, where the WT Apc antigen was lost through different pathways. Some tumor cells lack WT Apc and display strong cytoplasmic β-catenin, but not clusterin. This result indicates that neither loss of Apc function nor increased cytoplasmic β-catenin directly induces clusterin expression.
Tumor cells are normally poorly differentiated (23) during uncontrolled proliferation. Lack of differentiation markers in most tumor cells with elevated clusterin expression suggests that clusterin may be one marker for intestinal tumor cells. Nevertheless, a small number of cells do not fit this paradigm and either displayed both clusterin and differentiation markers or neither. Thus, clusterin cannot serve solo but must be joined into a set of tumor markers. In various models under different circumstances, clusterin may exert antiapoptotic or proapoptotic activities (14). Our observations are consistent with an antiapoptotic function of clusterin: the tumor cells with high apoptotic index are commonly deficient in clusterin expression.
The correlations of the expression pattern of clusterin with other cellular events suggested potential functions of clusterin in intestinal tumors. By introducing the Min allele into viable clusterin-deficient mice (46), the functional impact of clusterin expression on intestinal tumorigenesis can be investigated in the Min mouse model.
The mouse-generated candidate markers must be validated on human material for further use in the detection of colon cancer. High levels of RNase activity in colonic tissue make it challenging to fix macroscopic human biopsy samples without loss of RNA signal. The reliability of in situ hybridization with these samples requires further investigation. Alternatively, we have used immunofluorescence assays to validate clusterin production in the human tumor samples, because protein antigens are relatively stable and more directly coupled to analytical chemistry through radioimmunoassay or mass spectrometry. Elevated clusterin production in the human colonic tumors matched that in the mouse. The measurable elevation of antigen production in adenomatous polyps indicated that up-regulation of clusterin occurs very early in human colonic neoplasms. Staining of clusterin protein in the apical cytoplasm of tumor cells and in the intercellular cavities suggests that this protein may be secreted from the tumor cells. The comparison between murine and human adenocarcinomas was intriguing. By in situ hybridization, the murine adenocarcinoma showed strong elevation of clusterin mRNA in the invasive tumor cells. By immunofluorescence assay, the human adenocarcinoma showed only a slight increase in clusterin antigen production. Further studies are needed to ascertain whether these differences are fundamental or a reflection of the different detection methods.
The high level of clusterin antigen in the apical cytoplasm of morphologically normal human colon crypts adjacent to the adenomas or adenocarcinomas, but not in normal crypts far removed from the tumors or in tumor-free colonic tissues, was unexpected. We consider two hypotheses: tumor cells may release a paracrine factor that stimulates neighboring normal crypts to overexpress clusterin, or the secreted clusterin protein released from tumor cells may bind to the neighboring normal crypt epithelial cells and then be endocytosed into the apical cytoplasm of the recipient cells.
Clusterin antigen seems to be a sensitive and stable histological marker for murine and human intestinal tumors. This property makes clusterin a potential indicator for diagnosis of human colorectal cancer. Furthermore, elevated production of clusterin antigen, if secreted from tumor cells, may be detected in body fluids, such as serum. The intracellular polypeptide may also be detected from the exfoliated tumor cells contained in stool samples. It is important to investigate whether markers derived from clusterin can be used as part of a set of indices for early detection of human colorectal cancer.
Supplementary Material
Acknowledgments
We thank Cheri Pasch and Natalie Borenstein for PCR genotyping; Linda Clipson for major help in preparation of the manuscript; the histology core facility of the McArdle Laboratory for the preparation of histological samples; Chris Iacobuzio-Donahue at The Johns Hopkins University for providing nonradioactive in situ hybridization protocol in advance of publication; Michael Griswold at Washington State University (Pullman) for providing clusterin antibody; Erin Ruane and the McArdle animal care staff for animal husbandry; and Alexandra Shedlovsky for suggestions and discussion. This study was supported by National Institutes of Health Grants CA63677 and U01-CA84227 (to W.F.D.) and a postdoctoral fellowship for cancer prevention from the Cancer Research Foundation of America (to X.C.).
Abbreviations: B6, C57BL/6J; Min, multiple intestinal neoplasia, used as a genotypic abbreviation for ApcMin/+; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling.
References
- 1.American Cancer Society (2003) Cancer Facts & Figures 2003 (Am. Cancer Soc., Atlanta).
- 2.Beart, R. W. (1995) in Clinical Oncology, eds. Abeloff, M. D., Armitage, J. O., Lichter, A. S. & Niederhuber, J. E. (Livingstone, New York), pp. 1267-1286.
- 3.Neibergs, H. L., Hein, D. W. & Spratt, J. S. (2002) J. Surg. Oncol. 80, 204-213. [DOI] [PubMed] [Google Scholar]
- 4.Saffran, D. C., Reiter, R. E., Jakobovits, A. & Witte, O. N. (1999) Cancer Metastasis Rev. 18, 437-449. [DOI] [PubMed] [Google Scholar]
- 5.Burchardt, M., Burchardt, T., Shabsigh, A., de la Taille, A., Benson, M. C. & Sawczuk, I. (2000) Clin. Chem. 46, 595-605. [PubMed] [Google Scholar]
- 6.Stoll, B. A. (1999) Eur. J. Cancer 35, 693-697. [DOI] [PubMed] [Google Scholar]
- 7.Dorfinger, K., Kratzik, C., Madersbacher, S., Dorfinger, G., Berger, P. & Marberger, M. (1997) J. Urol. 158, 851-855. [DOI] [PubMed] [Google Scholar]
- 8.Su, L. K., Kinzler, K. W., Vogelstein, B., Preisinger, A. C., Moser, A. R., Luongo, C., Gould, K. A. & Dove, W. F. (1992) Science 256, 668-670. [DOI] [PubMed] [Google Scholar]
- 9.Moser, A. R., Pitot, H. C. & Dove, W. F. (1990) Science 247, 322-324. [DOI] [PubMed] [Google Scholar]
- 10.Dove, W. F., Cormier, R. T., Gould, K. A., Halberg, R. B., Merritt, A. J., Newton, M. A. & Shoemaker, A. R. (1998) Philos. Trans. R. Soc. London B 353, 915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich, W. F., Lander, E. S., Smith, J. S., Moser, A. R., Gould, K. A., Luongo, C., Borenstein, N. & Dove, W. F. (1993) Cell 75, 631-639. [DOI] [PubMed] [Google Scholar]
- 12.Iacobuzio-Donahue, C. A., Argani, P., Hempen, P. M., Jones, J. & Kern, S. E. (2002) Cancer Res. 62, 5351-5357. [PubMed] [Google Scholar]
- 13.Cormier, R. T., Hong, K. H., Halberg, R. B., Hawkins, T. L., Richardson, P., Mulherkar, R., Dove, W. F. & Lander, E. S. (1997) Nat. Genet. 17, 88-91. [DOI] [PubMed] [Google Scholar]
- 14.Trougakos, I. P. & Gonos, E. S. (2002) Int. J. Biochem. Cell Biol. 34, 1430-1448. [DOI] [PubMed] [Google Scholar]
- 15.Shoemaker, A. R., Moser, A. R., Midgley, C. A., Clipson, L., Newton, M. A. & Dove, W. F. (1998) Proc. Natl. Acad. Sci. USA 95, 10826-10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haigis, K. M. & Dove, W. F. (2003) Nat. Genet. 33, 33-39. [DOI] [PubMed] [Google Scholar]
- 17.Haigis, K. M., Caya, J. G., Reichelderfer, M. & Dove, W. F. (2002) Proc. Natl. Acad. Sci. USA 99, 8927-8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell, S. M., Zilz, N., Beazer-Barclay, Y., Bryan, T. M., Hamilton, S. R., Thibodeau, S. N., Vogelstein, B. & Kinzler, K. W. (1992) Nature 359, 235-237. [DOI] [PubMed] [Google Scholar]
- 19.Hugh, T. J., Dillon, S. A., O'Dowd, G., Getty, B., Pignatelli, M., Poston, G. J. & Kinsella, A. R. (1999) Int. J. Cancer 82, 504-511. [DOI] [PubMed] [Google Scholar]
- 20.Kishida, S., Yamamoto, H., Ikeda, S., Kishida, M., Sakamoto, I., Koyama, S. & Kikuchi, A. (1998) J. Biol. Chem. 273, 10823-10826. [DOI] [PubMed] [Google Scholar]
- 21.Behrens, J., Jerchow, B. A., Wurtele, M., Grimm, J., Asbrand, C., Wirtz, R., Kuhl, M., Wedlich, D. & Birchmeier, W. (1998) Science 280, 596-599. [DOI] [PubMed] [Google Scholar]
- 22.Mintz, B. & Fleischman, R. A. (1981) Adv. Cancer Res. 34, 211-278. [DOI] [PubMed] [Google Scholar]
- 23.Riedel, H. (2002) in The Cancer Handbook, ed. Alison, M. R. (Nature Publishing Group, London), pp. 953-970.
- 24.Moser, A. R., Dove, W. F., Roth, K. A. & Gordon, J. I. (1992) J. Cell Biol. 116, 1517-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavrieli, Y., Sherman, Y. & Ben Sasson, S. A. (1992) J. Cell Biol. 119, 493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byers, R. J., Hoyland, J. A., Dixon, J. & Freemont, A. J. (2000) Int. J. Exp. Pathol. 81, 391-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisitsyn, N., Lisitsyn, N. & Wigler, M. (1993) Science 259, 946-951. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, L., Zhou, W., Velculescu, V. E., Kern, S. E., Hruban, R. H., Hamilton, S. R., Vogelstein, B. & Kinzler, K. W. (1997) Science 276, 1268-1272. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg, M. E. & Silkensen, J. (1995) Int. J. Biochem. Cell Biol. 27, 633-645. [DOI] [PubMed] [Google Scholar]
- 30.de Silva, H. V., Harmony, J. A., Stuart, W. D., Gil, C. M. & Robbins, J. (1990) Biochemistry 29, 5380-5389. [DOI] [PubMed] [Google Scholar]
- 31.Wong, P., Pineault, J., Lakins, J., Taillefer, D., Leger, J., Wang, C. & Tenniswood, M. (1993) J. Biol. Chem. 268, 5021-5031. [PubMed] [Google Scholar]
- 32.Tenniswood, M., Wang, Z., Lakins, J., Morrissey, C., O'Sullivan, J. & Tang, H. (1998) J. Androl. 19, 508-516. [PubMed] [Google Scholar]
- 33.Jenne, D. E. & Tschopp, J. (1992) Trends Biochem. Sci. 17, 154-159. [DOI] [PubMed] [Google Scholar]
- 34.Jones, S. E. & Jomary, C. (2002) Int. J. Biochem. Cell Biol. 34, 427-431. [DOI] [PubMed] [Google Scholar]
- 35.Poon, S., Easterbrook-Smith, S. B., Rybchyn, M. S., Carver, J. A. & Wilson, M. R. (2000) Biochemistry 39, 15953-15960. [DOI] [PubMed] [Google Scholar]
- 36.Bach, U. C., Baiersdorfer, M., Klock, G., Cattaruzza, M., Post, A. & Koch-Brandt, C. (2001) Exp. Cell Res. 265, 11-20. [DOI] [PubMed] [Google Scholar]
- 37.Bartl, M. M., Luckenbach, T., Bergner, O., Ullrich, O. & Koch-Brandt, C. (2001) Exp. Cell Res. 271, 130-141. [DOI] [PubMed] [Google Scholar]
- 38.Wright, W. E. & Shay, J. W. (2001) Curr. Opin. Genet. Dev. 11, 98-103. [DOI] [PubMed] [Google Scholar]
- 39.Gonos, E. S., Derventzi, A., Kveiborg, M., Agiostratidou, G., Kassem, M., Clark, B. F., Jat, P. S. & Rattan, S. I. (1998) Exp. Cell Res. 240, 66-74. [DOI] [PubMed] [Google Scholar]
- 40.Marinelli, M., Quaglino, D., Bettuzzi, S., Strocchi, P., Davalli, P. & Corti, A. (1994) Biochem. Cell Biol. 72, 515-521. [DOI] [PubMed] [Google Scholar]
- 41.Yang, C. R., Leskov, K., Hosley-Eberlein, K., Criswell, T., Pink, J. J., Kinsella, T. J. & Boothman, D. A. (2000) Proc. Natl. Acad. Sci. USA 97, 5907-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinzler, K. W. & Vogelstein, B. (1996) Cell 87, 159-170. [DOI] [PubMed] [Google Scholar]
- 43.Halberg, R. B., Katzung, D. S., Hoff, P. D., Moser, A. R., Cole, C. E., Lubet, R. A., Donehower, L. A., Jacoby, R. F. & Dove, W. F. (2000) Proc. Natl. Acad. Sci. USA 97, 3461-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liefers, G. J. & Tollenaar, R. A. (2002) Eur. J. Cancer 38, 872-879. [DOI] [PubMed] [Google Scholar]
- 45.Yang, V. W. (2002) Gastroenterology 123, 935-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLaughlin, L., Zhu, G., Mistry, M., Ley-Ebert, C., Stuart, W. D., Florio, C. J., Groen, P. A., Witt, S. A., Kimball, T. R., Witte, D. P., et al. (2000) J. Clin. Invest. 106, 1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.