Abstract
Beyond the key role in reproductive and cognitive functions, estrogens have been shown to protect against neurodegeneration associated with acute and chronic injuries of the adult brain. Current hypotheses reconcile this activity with a direct effect of 17β-estradiol (E2) on neurons. Here we demonstrate that brain macrophages are also involved in E2 action on the brain. Systemic administration of hormone prevents, in a time- and dose-dependent manner, the activation of microglia and the recruitment of peripheral monocytes induced by intraventricular injection of lipopolysaccharide. This effect occurs by limiting the expression of neuroinflammatory mediators, such as the matrix metalloproteinase 9 and lysosomal enzymes and complement C3 receptor, as well as by preventing morphological changes occurring in microglia during the inflammatory response. By injecting lipopolysaccharide in estrogen receptor (ER)-null mouse brains, we demonstrate that hormone action is mediated by activation of ERα but not of ERβ. The specific role of ERα is further confirmed by comparing the effects of ERs on the matrix metalloproteinase 9 promoter activity in transient transfection assays. Finally, we report that genetic ablation of ERα is associated with a spontaneous reactive phenotype of microglia in specific brain regions of adult ERα-null mice. Altogether, these results reveal a previously undescribed function for E2 in brain and provide a mechanism for its beneficial activity on neuroinflammatory pathologies. They also underline the key role of ERα in brain macrophage reactivity and hint toward the usefulness of ERα-specific drugs in hormone replacement therapy of inflammatory diseases.
Estrogen replacement therapy in postmenopausal women is associated with a decrease in cognitive deterioration, depression, and psychosis (1–3) and with a delayed onset of Alzheimer's disease (AD), a chronic neurodegenerative disorder (4–6). In addition, the severity of multiple sclerosis, a progressive destructive disease of neurons, remits during pregnancy and relapses postpartum, when female steroid hormones are at high and low levels, respectively (7, 8), whereas estrogen administration after cerebral stroke is also associated with decreased mortality and morbidity (9). The efficacy of estrogen during or after brain injury is also well documented by several models of neurodegeneration and ischemia. In ovariectomized rats, estradiol administration attenuates the extent of brain damage caused by permanent or transient brain ischemia (10, 11) and reduces cortical lesions due to glutamate excitotoxicity (12) or secondary to status epilepticus (13), autoimmunity (14), and demyelinating diseases (15, 16). These clinical and experimental data suggested that estrogens are protective factors for the adult mammalian brain and powerful pharmacological tools for the prevention of diverse neurological disorders. In most studies substantiating this concept, the emphasis has been on neuron survival and plasticity (17). However, a growing number of studies point to alternative mechanisms involving membrane-coupled estrogen receptors (ERs) or antioxidant properties of estradiol when used at pharmacological concentrations, i.e., in the micromolar range (18).
Increasing evidence highlights the role played by inflammation and brain macrophages in the pathogenesis and progression of neurological disorders. Microglia, the macrophage cells of the brain, regulate brain homeostasis and defense against mechanical or chemical injury by mounting the inflammatory response (19). A state of chronic microglia activation has been observed in several neurodegenerative diseases and correlated with neuron loss and matrix destruction, which are involved in the mechanism of neurodegeneration (20, 21). Furthermore, macrophages invading focal regions of the brain and spinal cord of multiple sclerosis patients take an active part in the early stages of axon demyelination and plaque formation and, together with activated microglial and T cells, sustain the inflammatory response and associated disability (22). The negative role played by inflammation on neuron loss is substantiated by recent findings showing that nonsteroidal antiinflammatory drugs are efficacious in reducing the incidence and progression of AD (23).
We previously reported that macrophage cells are targets of 17β-estradiol (E2) action (24). Further investigations in our laboratory and those of others have shown that activation by E2 of endogenous ERs, which are hormone-regulated transcription factors (25), leads to a significant inhibition of microglial reactivity in vitro (26, 27). Using experimentally induced brain inflammation, we demonstrate here that physiological concentrations of E2 inhibit brain macrophage reactivity and show that the ERα isotype regulates this activity. According to these results, we report that genetic ablation of ERα is associated with a spontaneous reactive phenotype of microglia in adult ERα-null mice.
Materials and Methods
Animals. The study was conducted according to the guidelines of the Institutional Animal Care Committee of the University of Milan. ERα and -β-null mice have a C57B6 genetic background. Generation of these animals has been described (28).
Intracerebroventricular Injections of Lipopolysaccharide (LPS) and Hormone Treatment. Four-week-old Sprague–Dawley female rats (Charles River Breeding Laboratories) were ovariectomized and used 2 wk later for the experiments. Intact female ERα- and -β-null mice and the respective wild-type littermates were used at 8–10 weeks of age. Animals were injected s.c. with vehicle (vegetal oil) or 50 μg/kg E2 (Sigma) 6 h before the injection, under deep anesthesia, of Escherichia coli LPS [serotype 0.111:B4 (Sigma); for rats: 10 μg of LPS in 3 μl of saline; for mice: 1 μg of LPS in 1 μl of saline) in the third cerebral ventricle (icv) according to specific stereotaxic coordinates (for rats: bregma, -4 mm; lateral, 1 mm; depth, 5 mm; for mice: bregma, -0.25 mm; lateral, 1 mm; depth, 2.25 mm), as described (29, 30). After 24 h, animals were anesthetized and transcardially perfused with 4% paraformaldehyde; brains were postfixed, cryo-protected, snap-frozen in liquid nitrogen, and stored at -80°C. No alteration in animal behavior or body temperature and no acute-phase proinflammatory proteins in plasma were detected (data not shown). Experiments were repeated at least three times; each experimental group consisted of three to five animals.
Immunohistochemistry. Thirty-micrometer brain coronal sections were collected from bregma by using a cryostat (from Microm, Walldorf, Germany) and analyzed free-floating with immunohistochemistry by using horseradish peroxidase (HRP)-conjugated isolectin-B4 (Sigma), ED-1 (Serotec), Mac-1 [for complement-3 receptor (C3R); Serotec], and matrix metalloproteinase-9 (MMP-9; from Chemicon). An antigen–antibody complex was detected with the avidin-biotin-HRP kit (Vector Laboratories) and 3,3′-diaminobenzidine (Sigma). Dehydrated sections mounted on slides were observed by using a Zeiss Axioskop Microscope (Zeiss); microphotographs were taken in the contralateral hemisphere by using a 990 Coolpix digital camera (Nikon) and printed in black and white by using standard computer programs. Some sections were counterstained with 0.5% methyl green. An ERα-null microglia phenotype was evaluated on coronal sections -2.75 mm from bregma.
Quantitative Analysis. ED-1-positive cells in coronal sections ± 180 μm from the injection site were counted in areas of defined size: CA3, 2.2 mm2; dentate gyrus (DG), 0.9 mm2; cingulate cortex, 1.2 mm2; parietal cortex, 7 mm2; posterolateral and posteromedial cortical amygdaloid nuclei, 1 mm2; posterolateral thalamic nuclei, 1,1 mm2; rhinal cortex, 2.3 mm2. MMP-9- and Mac-1-positive cells were counted in 0.12 mm2 of the CA1 region on coronal sections at -2.5 mm from mouse brain bregma. An average number of cells were obtained from three coronal sections of a single animal; no variation of statistical significance could be found between individuals of the same experimental groups.
RT-PCR. RNA extraction from whole mouse brain, reverse transcription, and PCRs was performed as described (27). Amplification of MMP-9 and glyceraldehyde phosphodehydrogenase, a constitutively expressed gene used to normalize for reaction efficiency, resulted in 293- and 625-bp-long products, respectively. PCRs were run by using a Thermal Cycler 480 (Perkin–Elmer).
Cell Culture and Transfections. HeLa cells were grown at 37°C in RPMI medium 1640 (Sigma) supplemented with 10% FBS/1% nonessential amino acids/5 mM l-glutamine/100 μg/ml streptomycin–penicillin, at 37°C under a humidified 5% CO2/95% air atmosphere. For transient transfection experiments, 105 cells per well were seeded in 24-well plates in 500 μl of RPMI medium 1640 without phenol red (Sigma) with 10% charcoal-treated FBS. Transient transfections were performed as described (27). The reported luciferase activity was calculated by normalizing by protein content. Each experiment was performed on triplicate samples and repeated at least three times.
Data Analysis. Values are given as mean ± SD. P values were calculated with ANOVA analysis followed by the Bonferroni test.
Results
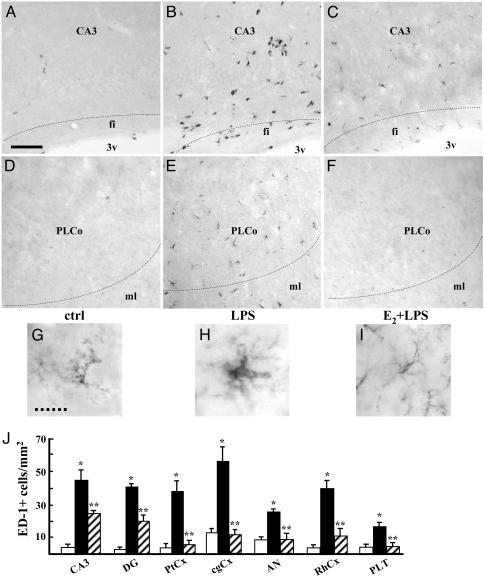
Estradiol Inhibits Microglial Reactivity and Monocyte Recruitment in the Brain. Injection of LPS in the rat third cerebral ventricle leads to the activation of brain macrophages, namely microglial and blood-derived monocytes. Macrophage activation is generally characterized by the synthesis of phagocytotic and inflammatory mediators, including lysosomal and matrix-degrading enzymes and complement components, and by morphological changes. Therefore, we first assessed the effect of LPS and E2 on brain macrophages by analyzing the expression of the ED-1 lysosomal protein in the hippocampus. Expression and glycosylation of ED-1 antigen are enhanced by inflammatory stimuli, similarly to other lysosome-associated membrane proteins, and linked to phagocytosis (31). We chose to inject the hormone 6 h before LPS, because this is the shortest time interval necessary for full induction of estrogen activity in vivo (32). As expected, ED-1 expression was induced by LPS in both resident microglia and monocytes recruited from the blood stream (Fig. 1 A and B), whereas E2 alone did not modify any parameter of macrophage activation (data not shown); however, LPS-mediated induction of ED-1 expression was strongly inhibited by systemic administration of E2 before the endotoxin (Fig. 1C). A similar effect of hormone and LPS on ED-1 expression was observed in brain macrophages in the frontal and parietal cortices, whereas in the amygdala, rhinal cortex, and thalamus, LPS led to microglia activation without monocyte recruitment. To evaluate the effect of E2 on the morphologic changes associated with cell activation, we used Isolectin-B4, which stains both resting and activated microglial cells. From typical ramified cell morphology, microglia switched to a reactive state, characterized by an increase in cell body size with shorter and thicker cell processes (compare Fig. 1 D and E). E2 administration clearly prevented this LPS-induced morphologic reactivity (Fig. 1F). To quantify the hormonal effect, we counted the number of ED-1-positive cells and showed that E2 reduced LPS activity by 45% in the hippocampus (CA3 and DG); by 95% in the parietal and cingulate cortices, amygdala and thalamus; and by 76% in the rhinal cortex (Fig. 1G). Altogether, these results indicate that E2 opposes LPS action by impairing microglia activation throughout the temporal lobe and the limbic system of rat brain and by inhibiting the recruitment of circulating monocytes that occurs in the hippocampus and temporal cortex after icv LPS.
Fig. 1.
Estrogen prevents activation of brain macrophages induced by LPS. Shown are representative immunohistochemical assays with ED-1 as a marker for microglia and monocytes in the CA3 field of the hippocampus (A–C) and in posterolateral cortical amygdaloid nuclei (D–F); animals were injected with saline (A and D), LPS (B and E), or E2 before LPS (C and F). CA3, CA3 field of the hippocampus; fi, fimbria; 3v, third ventricle. PLCo, posterolateral cortical amygdaloid nuclei; ML, molecular layer. (Bar = 35 μm.) (G–I) Isolectin-B4 was used to visualize microglial morphology in the CA3 region of animals treated as specified. (Dashed bar = 8 μm.) (J) The number of ED-1-positive microglial cells was counted in the CA3 and DG of the hippocampus, in the parietal cortex (PtCx), cingulate cortex (cgCx), amygdaloid nuclei (AN), rhinal cortex (RhCx), and posterolateral thalamic nuclei (PLT). Saline, LPS, and E2 + LPS treatments are represented as open, filled, and dashed bars, respectively. Values are the mean ± SD of cells counted in adjacent sections from a single representative experiment, repeated at least three times. *, P < 0.01 vs. control; **, P < 0.01 vs. LPS.
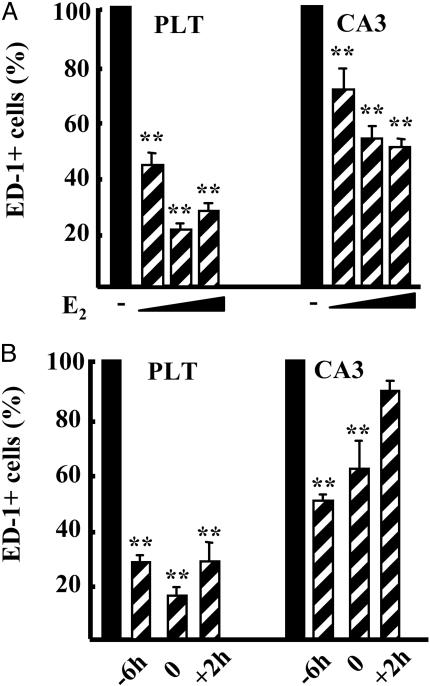
Inhibition of Brain Macrophage Reactivity Correlates with the Activation of Endogenous Receptors. To understand whether the effect of hormone could be ascribed to the interaction with specific endogenous receptors, we used increasing doses of E2. In the thalamus, a dose of 0.5 μg/kg E2 was sufficient to reach 55% inhibition of LPS activity; 5 and 50 μg/kg resulted in a further inhibition up to 80% (Fig. 2A). In the CA3 region, E2 action resulted in a similar dose-dependent profile of activity, albeit with a lower efficacy, because 0.5 μg/kg E2 resulted in 28% inhibition of LPS activity, and further increased with higher doses of E2 to reach 50% inhibition (Fig. 2 A). It is worth mentioning that injection of 5 μg/kg E2 resulted in circulating levels of hormone similar to the physiological range (5 pg/ml), and that the dose–response curve of E2 action on macrophage reactivity was superimposable to that of ER transcriptional activity measured in ERE-Luc mice (32), suggesting that ERs are involved in hormone action on brain macrophages in vivo.
Fig. 2.
Time- and dose-dependent inhibitory effect of estrogen on brain macrophage activation. (A) The number of ED-1-positive cells in the posterolateral thalamus (PLT) and CA3 was counted in rat brains injected with LPS 6 h after s.c. injection of vehicle (filled bars) or increasing doses of E2 (0.5, 5, and 50 μg/kg of E2; dashed bars). (B) Fifty micrograms/kilogram E2 injected 6 h before (-6h), at the same time as (0), or 2 h after (+2h) LPS. Bars are the average ± SD of a single representative experiment made on three animals; the experiment was repeated three times. Data are calculated as percentage of LPS alone. **, P < 0.01 vs. LPS.
We then assayed different time intervals between hormone and LPS injections. Microglia activation by LPS in the thalamus was impaired by E2 injected either 6 h before, at the same time as, or 2 h after the endotoxin (Fig. 2B). When we analyzed the CA3 region, we observed that the 2-h time frame was not sufficient for hormone to counteract the recruitment of monocytes induced by LPS, suggesting that this process is less responsive to hormone when compared with microglia reactivity; similar results were observed in other brain regions involving microglia and monocytes or microglia alone (data not shown).
Thus, doses of E2 leading to physiological levels of hormone are sufficient to cause brain macrophage inhibition. These data suggest that estrogen action on brain inflammation is mediated by specific receptors, which share activation profiles similar to those of the classical intracellular receptors.
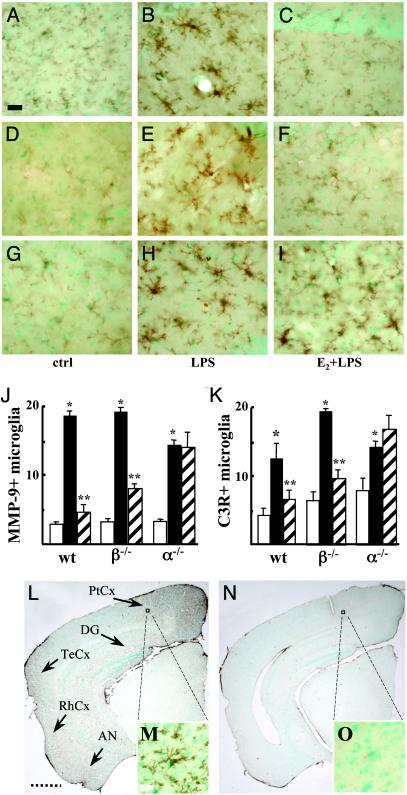
ERα Controls Microglia Reactivity. We then investigated the possible role played by ERα and/or ERβ using ER-null mice. Brain reactive macrophages were identified by using antibodies against MMP-9 and C3R; these proteins are known to be involved in the inflammatory response driven by microglia and other macrophage cells. In fact, the role of MMPs in the degradation of the basal lamina around the cerebral vessels and in the promotion of leukocyte extravasation is well documented in multiple phases of neuroinflammation and demyelination (33); on the other hand, C3R belongs to the integrin family that mediates leukocyte recruitment and blood–brain barrier permeability and, together with their ligands, is found up-regulated during CNS inflammation and is associated with dystrophic neurons in AD (34). Therefore, we assessed the expression of MMP-9 and C3R to evaluate the effect of E2 on the functional activation of brain macrophages in response to LPS. When compared with rats, wild-type female mice injected icv with LPS showed a more restricted distribution of microglia activation and a lack of monocyte infiltration, as reported (30, 35). Consistent with the effect of the hormone in rats, LPS-induced brain expression of MMP-9 was severely impaired by E2 (Fig. 3 A–C). Data from wild-type littermates of the ERα family are shown in Fig. 3; however, similar results were obtained by using wild-type mice from the ERβ family (data not shown). We also observed that the reactivity of microglia in response to either LPS or E2 + LPS in wild-type mice is not influenced by the stage of the estrous cycle (data not shown). The response of ERβ-null mice to LPS alone or after E2 injection was similar to that observed in wild-type animals (Fig. 3 D–F), suggesting that ERβ is dispensable for E2-dependent inhibition of microglia activation. On the contrary, microglia were equally reactive to LPS in the absence or presence of E2 in ERα-null animals (Fig. 3 G–I). We chose to treat mice with 50 μg/kg E2 to achieve supraphysiological concentrations of hormone in the blood. Because it is known that ERα female mice have higher levels of circulating E2, the final hormone concentration reached by the pharmacological treatment minimizes any possible contribution of the endogenous hormone. To quantify the effect of hormone and LPS signaling, we counted MMP-9 and Mac-1-positive cells in the CA1 region of ER-null mice. The inhibitory activity of E2 was similar in wild-type and ERβ-null animals, suggesting that the absence of ERβ did not modify microglia reactivity, whereas the hormone did not inhibit the effect of LPS in ERα-null mice (Fig. 3 J and K). Similar effects of hormone on microglia morphology were obtained in primary cultures of microglia extracted from ERβ- and -α-null mice (data not shown). These results demonstrate that ERα, but not ERβ, is indispensable for estrogen blockade of brain macrophage reactivity in vivo, and that ERα mediates E2 signaling on both the morphologic and biochemical activation of microglia.
Fig. 3.
ER-α mediates the inhibitory activity of E2 on microglia activation. MMP-9 expression in the CA1 region of wild-type (A–C), ERβ-null (D–F), and ERα-null (G–I) mice injected icv with saline (A, D, and G), LPS (B, E, and H), or E2 before LPS (C, F, and I). (J and K) Quantification of estrogen activity on the number of MMP-9-positive (J) and C3R-positive (K) cells per mm2 in the CA1 region of mice with different ER genetic background. Saline, LPS, and E2 + LPS treatments are represented as open, filled, and dashed bars, respectively. Bars are from a single representative experiment, repeated at least three times. Values represent the mean ± SD of cells counted in different adjacent sections. *, P < 0.01 vs. control; **, P < 0.01 vs. LPS. (L–O) Disruption of ERα results in brain microglia activation. Microglial cells were visualized by immunohistochemistry of C3R on brain coronal sections of ERα-null mice at 4 mo (L and M) or 2 mo (N and O) of age. (L) Strong C3R staining shows a reactive phenotype in the parietal cortex (PtCx), DG, temporal cortex (TeCx), rhinal cortex (RhCx), and amygdaloid nuclei (AN; see arrows). (M and O) Enlargements showing the reactive phenotype of microglia only in a 4-mo-old ERα-null mouse. Sections were counterstained with methyl green. (Filled bars = 35 μm, dashed bars = 1.2 mm, and open bars = 7 μm.)
The specific role of ERα in brain inflammatory response is further sustained by the following observation. We analyzed whether the mildly reactive phenotype of control ERα-null animals, observed in the icv experiment (Fig. 3K), could evolve with age. Interestingly, we observed sustained microglia activation in 4-mo-old ERα-null mice as compared with younger animals (Fig. 3 L–O). In particular, C3R immunolabeling revealed the presence of reactive microglia cells in the hippocampus (CA1 region and DG), in the overlying cerebral cortex (cingulate and parietal), in the temporal and rhinal cortices, and in the amygdala (Fig. 3L). An average of 13 reactive microglia cells per mm2 were counted in these regions and appeared dispersed in the parenchyma without any propensity toward specific cortical layers. The mesencephalon showed a resting microglia morphology. Magnification of the histological sections showed that microglia cells are in a reactive state (Fig. 3M); in fact, C3R increased expression localized to cells with the typical phenotype of reactive microglia. Disruption of ERα leads to microglia activation also in male mice of similar age (data not shown), suggesting that the basal inflammatory phenotype is not gender-specific. Wild-type and ERβ-null mice did not show reactive microglia (data not shown).
Altogether, these phenotypic observations indicate that disruption of ERα leads to a spontaneous proinflammatory phenotype of microglia, thus demonstrating a pivotal role for this receptor in controlling the inflammatory status of the brain.
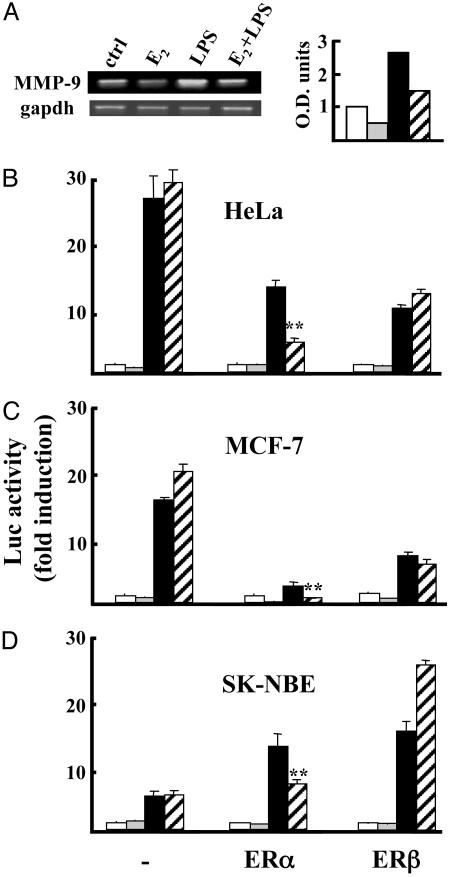
Inhibition of MMP-9 Expression by Estrogen Is Mediated by ERα. We further investigated the molecular details of the diversity between ERα and -β. By RT-PCR analysis on brain extracts from mice injected with LPS and/or E2, we demonstrated that hormonal treatment prevented the increase in MMP-9 mRNA levels induced by LPS (Fig. 4A). This observation suggested that the regulation of MMP-9 expression by LPS and hormone could be at the transcriptional level. To evaluate the contribution of each receptor isoform, transient transfection assays were performed by using a reporter of the MMP-9 gene promoter, along with ERα or -β expression vectors. Fig. 4 B–D shows that phorbol 12-myristate-13-acetate had a different potency on MMP-9 promoter transcription in the different cell lines tested; however, E2-activated ERα was able to prevent promoter induction in any cellular context, whereas ERβ either increased or did not modify promoter activity (Fig. 4 B–D). These results lend further support to the conclusion that ERα is specifically involved in the inhibition of MMP-9 gene expression driven by E2.
Fig. 4.
E2-activated ER-α inhibits the induction of MMP-9 expression. (A) RT-PCR detection of MMP-9 RNA levels in the brain. (Right) Optical density of the bands as normalized with GAPDH. Groups of three animals were treated with saline (ctrl, open bar), E2 alone (light gray bar), LPS (filled bar), or E2 + LPS (dashed bar). Data are from single animals representative of each experimental group; the experiment was repeated three times. (B) MMP-9 promoter regulation by ERs. HeLa (B), MCF-7 (C), and SK-NBE (D) cell lines were transfected with the MMP-9-luc promoter alone (-) or with ERα or ERβ and treated with vehicle (open bars), 10-9 M E2 (light gray bars), 10-8 M phorbol 12-myristate-13-acetate (PMA; filled bars), or E2+PMA (dashed bars). Values represent the mean ± SD of triplicate samples of a single experiment repeated at least three times. *, P < 0.01 vs. LPS.
Discussion
The present study demonstrates that physiological concentrations of estradiol prevent brain inflammatory response to the icv injection of a powerful inflammatory agent, LPS. We show that E2 treatment inhibits microglia activation, as demonstrated by the lack of acquisition of the typical reactive morphology of these cells, by the impaired expression of proteins associated with phagocytosis and cell migration, and by the reduced infiltration of leukocytes through the brain parenchyma. Thus, all of the steps of brain inflammatory reaction induced by LPS in ovariectomized rats are inhibited by hormone treatment. These results suggest that E2 might be necessary for limiting the excessive response to physiological inflammatory signals in the adult animal; in addition, our data may have relevant implications for explaining the protective effects of hormone described in several etiologically different CNS pathologies, such as multiple sclerosis (4–6), AD (7, 8), and transient brain ischemia (9). In fact, increased expression of C3R, MMP-9, and other inflammatory markers, together with reactive microglia and infiltrated leukocytes, is associated with neurodegenerative and demyelinating processes, which suggested that chronic inflammation is a key component in neurodegenerative pathologies of various etiology (20). Therefore, we propose a novel hypothesis to explain the beneficial effect of hormone in brain, which we suggest occurs by limiting the contribution of brain inflammatory cells to disease onset and progression. Interestingly, previous observations on experimental models of CNS inflammation and transient ischemia reported that E2 inhibits the synthesis of adhesion molecules on vascular cells (36–38), in line with the hypothesis of estrogen being a vasoactive agent in cardiovascular physiology (39). Thus, in addition to a direct effect on the vessel wall, our observations show that estrogen acts in brain to impair the contribution of microglia signaling pathways to the recruitment of leukocytes from the periphery. One should also consider that icv injection of LPS, as expected, produced a diffused pattern of brain inflammation and allowed us to evaluate that estrogen action is not restricted to a specific brain region. This further sustains our hypothesis, because neurodegenerative processes are characterized by different etiology and topographical distribution, whereas they share similar signs of macrophage activation.
With regard to the molecular mechanism of hormone action, our study shows that the antiinflammatory effect of E2 in microglial cells is mediated by ERα; this is a previously undescribed demonstration of a receptor-mediated mechanism of action of estrogens in macrophage cells of the brain, which was hypothesized previously only by using primary microglial cells in culture (27) or glial cell lines (26). Our observation that disruption of ERα by itself results in a spontaneous reactive phenotype of microglia further supports the role of this specific hormone receptor in inflammatory pathways in the brain. The mechanism of this age-dependent activation of microglia is still unknown. Because accumulation of reactive oxygen and nitrogen oxide species in the adult brain leads to an oxidative stress that activates microglia, it is possible that this event occurs earlier in the ERα-null mice, which lack the protective effects of hormone. Interestingly, the increased reactivity of microglia is localized to the same brain areas where neurodegeneration and reactive microglia have been observed in AD, such as the parietal layers overlying the hippocampus and the entorhinal and piriform cortices. This observation leads to speculation that microglial cells are more susceptible to sense proinflammatory signals in the brain areas described above. Further studies are needed to shed more light on these aspects.
Although we indicate in ERα the mediator of E2 signaling in brain inflammatory cells, considering the role played by this receptor in endothelial cells of cerebral vessels (40) and in neuronal death after ischemia (41), we do not exclude that ERβ might also be involved in regulating neural cell function and viability, as demonstrated (42).
Once activated by the endogenous hormone, ERs interact with specific transcriptional coregulators to modulate gene expression; in addition, a nuclear-independent activity of ER has been reconciled with the rapid effects evoked by the hormone, which have been attributed to the interaction with intracellular signaling pathways (43). We here show that induction of MMP-9 mRNA levels in the brain is inhibited by E2. When tested in transient transfection assays, this effect could be ascribed to the inhibitory transcriptional activity of ERα and not ERβ, which is entirely consistent with our results using ER-null mice. These results provide previously undescribed evidence for a different molecular mechanism of action of ERs on a natural promoter. Because we did not find any canonical estrogen responsive elements in the MMP-9 promoter sequence, we hypothesize that ERα acts without directly binding to DNA to interfere with the transcriptional activity of other proteins, such as the members of the AP-1 or Nf-kB families of transcription factors, which are known to be activated by LPS. This ERα-specific activity could be ascribed to different mechanisms, such as the activation by E2 of specific interaction surfaces on the two ERs, which creates distinct nuclear recognition capabilities, or the existence of receptor-specific cytoplasmic effectors, which control the LPS signaling pathway. Further studies are underway to unravel the differential mechanism of action of ERs in macrophage cells.
The identification of a specific role played by ERα in brain inflammatory pathways has relevant implications for a pharmacological perspective, because it indicates that ERα-selective agonists may represent more appropriate pharmacological tools for hormone replacement therapies of neuroinflammatory disorders. Because most prevalent pathologies associated with postmenopause, such as osteoporosis and atherosclerosis, are correlated with a local chronic inflammatory reaction (44), our data may suggest that ERα could be a pharmacological target also to mimic the beneficial effects of E2 on the onset and progression of these brain-unrelated pathologies (45).
Acknowledgments
We thank Cinzia Bonincontro, Paola Mussi, and Paolo Sparaciari for critical support; Monica Rebecchi and Samanta Oldoni for skillful technical assistance; and Elisabetta Gianazza, Elda Scherini, and Carlo Pellicciari for helpful discussion. This research was supported by grants from Telethon (no. GP0127Y01), Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (40%), the European Community (no. QLRT-2001-02221), and COFIN 58785. The work at Institut de Génétique et de Biologie Moléculaire et Cellulaire was supported by a grant from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale and the Collège de France.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AD, Alzheimer's disease; ER, estrogen receptor; E2, 17β-estradiol; MMP-9, matrix metalloproteinase 9; C3R, complement-3 receptor; DG, dentate gyrus; icv, in the third cerebral ventricle; LPS, lipopolysaccharide.
References
- 1.Sherwin, B. (2002) Trends Pharmacol. Sci. 23, 527-534. [DOI] [PubMed] [Google Scholar]
- 2.Freeman, M. P., Smith, K. W., Freeman, S. A., McElroy, S. L., Kmetz, G. E., Wright, R. & Keck, P. E., Jr. (2002) J. Clin. Psychiatry 63, 284-287. [DOI] [PubMed] [Google Scholar]
- 3.Huber, T. J., Rollnik, J., Wilhelms, J., von zur Muhlen, A., Emrich, H. M. & Schneider, U. (2001) Psychoneuroendocrinology 26, 27-35. [DOI] [PubMed] [Google Scholar]
- 4.Henderson, W. (1997) Neurology 48, S27-S35. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe, K., Sawaya, G., Lieberburg, I. & Grady, D. (1998) J. Am. Med. Assoc. 9, 688-695. [DOI] [PubMed] [Google Scholar]
- 6.Zandi, P. P., Carlson, M. C., Plassman, B. L., Welsh-Bohmer, K. A., Mayer, L. S., Steffens, D. C. & Breitner, J. C. (2002) J. Am. Med. Assoc. 288, 2123-2129. [DOI] [PubMed] [Google Scholar]
- 7.Confavreux, C., Hutchinson, M., Hours, M. M., Cortinovis-Tourniaiare, P. & Moreau, T. (1998) N. Engl. J. Med. 339, 285-291. [DOI] [PubMed] [Google Scholar]
- 8.Birk, K., Ford, C. & Meltzer, S. (1990) Arch. Neurol. 47, 738-742. [DOI] [PubMed] [Google Scholar]
- 9.Hurn, P. D. & Macrae, I. M. (2000) J. Cereb. Blood Flow Metab. 20, 631-652. [DOI] [PubMed] [Google Scholar]
- 10.Yang, S. H., Shi, J., Day, A. L. & Simpkin, J. W. (2000) Stroke 31, 745-749. [DOI] [PubMed] [Google Scholar]
- 11.Carswell, H. V., Dominicsak, A. F. & Mancrae, I. M. (2000) Am. J. Physiol. 278, H290-H294. [DOI] [PubMed] [Google Scholar]
- 12.Mendelowitsch, A., Ritz, M. F., Ros, J., Langemann, H. & Gratzl, O. (2001) Brain Res. 901, 230-236. [DOI] [PubMed] [Google Scholar]
- 13.Veliskova, J., Velisek, L., Galanopoulou, A. S. & Sperber, E. F. (2000) Epilepsia 41 (Suppl. 6), S30. [DOI] [PubMed] [Google Scholar]
- 14.Jansson, L. & Holmdahl, R. (1998) Inflamm. Res. 47, 290-301. [DOI] [PubMed] [Google Scholar]
- 15.Jansson, L., Olsson, T. & Holmdahl, R. (1994) J. Neuroimmunol. 53, 203-207. [DOI] [PubMed] [Google Scholar]
- 16.Offner, H., Adlard, K., Zamora, A. & Vanderbark, A. A. (2000) J. Clin. Invest. 105, 1465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toran-Allerand, C. D., Singhm, M. & Setalo, G., Jr. (1999) Front. Neuroendocrinol. 20, 97-121. [DOI] [PubMed] [Google Scholar]
- 18.Stefano, G. B., Prevot, V., Beauvillain, J. C., Fimiani, C., Welters, I., Cadet, P., Breton, C., Pestel, J., Salzet, M. & Bilfinger, T. V. (1999) J. Immunol. 163, 378-383. [PubMed] [Google Scholar]
- 19.Kreutzberg, G. W. (1996) Trends Neurosci. 19, 312-318. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Scarano, F. & Baltuch, G. (1999) Annu. Rev. Neurosci. 22, 219-240. [DOI] [PubMed] [Google Scholar]
- 21.Kalaria, R. N. (1999) Curr. Opin. Hematol. 6, 15-24. [DOI] [PubMed] [Google Scholar]
- 22.Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Bruck, W. (2000) Brain 123, 1174-1183. [DOI] [PubMed] [Google Scholar]
- 23.Aisen, P. S. (2992) Lancet Neurol. 1, 279-284. [DOI] [PubMed] [Google Scholar]
- 24.Vegeto, E., Pollio, G., Pellicciari, C. & Maggi, A. (1999) FASEB J. 13, 793-803. [DOI] [PubMed] [Google Scholar]
- 25.Mueller, S. O. & Korach, K. S. (2001) Curr. Opin. Pharmacol. 1, 613-619. [DOI] [PubMed] [Google Scholar]
- 26.Bruce-Keller, A. J., Barger, S. W., Moss, N. I., Pham, J. T., Keller, J. N. & Nath, A. (2001) J. Neurochem. 78, 1315-1324. [DOI] [PubMed] [Google Scholar]
- 27.Vegeto, E., Bonincontro, C., Pollio, G., Sala, A., Viappiani, S., Nardi, F., Brusadelli, A., Viviani, B., Ciana, P. & Maggi, A. (2001) J. Neurosci. 21, 1908-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupont, S., Krust, A., Gansmuller, A., Dierich, A., Chambon, P. & Mark, M. (2000) Development (Cambridge, U.K.) 127, 4277-4291. [DOI] [PubMed] [Google Scholar]
- 29.Hauss-Wegrzyniak, B., Lukovic, L., Bigaud, M. & Stoeckel, M. E. (1998) Brain Res. 794, 211-224. [DOI] [PubMed] [Google Scholar]
- 30.Andersson, P.-B., Perry, V. H. & Gordon, S. (1992) Neuroscience 48, 169-186. [DOI] [PubMed] [Google Scholar]
- 31.Damoiseaux, J. G. M. C., Dopp, E. A., Calaìme, W., Chao, D., MacPherson, G. G. & Dijkstra, C. D. (1994) Immunology 83, 140-147. [PMC free article] [PubMed] [Google Scholar]
- 32.Ciana, P., Raviscioni, M., Mussi, P., Vegeto, E., Que, I., Parker, M. G., Lowik, C. & Maggi, A. (2002) Nat. Med. 9, 82-86. [DOI] [PubMed] [Google Scholar]
- 33.Yong, V. W., Power, C., Forsyth, P. & Edwards, D. R. (2001) Nat. Rev. Neurosci. 2, 502-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archelos, J. J., Previatli, S. C. & Hartung, H. P. (1999) Trends Neurosci. 22, 30-38. [DOI] [PubMed] [Google Scholar]
- 35.Lawson, L. J., Perry, V. H., Dri, P. & Gordon, S. (1990) Neuroscience 39, 151-170. [DOI] [PubMed] [Google Scholar]
- 36.Ito, A., Buenafe, A. C., Matejuk, A., Zamora, A., Silverman, M., Dwyer, J., Vandenbark, A. A. & Offner, H. (2002) Clin. Immunol. 102, 275-282. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda, J., Vanier, M. T., Saio, Y., Suzuki, K. & Suzuki, K. (2001) Hum. Mol. Genet. 10, 2709-2715. [DOI] [PubMed] [Google Scholar]
- 38.Santino, R. A., Koenig, H. M. & Pellegrino, D. A. (2000) Stroke 31, 2231-2235. [DOI] [PubMed] [Google Scholar]
- 39.Koh, K. K. (2002) Cardiovasc. Res. 55, 714-726. [DOI] [PubMed] [Google Scholar]
- 40.Geary, G. G., McNeill, A. M., Ospina, J. A., Krause, D. N., Korach, K. S. & Duckeles, S. P. (2001) J. Appl. Physiol. 91, 2391-2399. [DOI] [PubMed] [Google Scholar]
- 41.Dubal, D. B., Zhu, H., Yu, J., Rau, S. W., Shughrue, P. J., Merchenthaler, I., Kindy, M. S. & Wise, P. M. (2001) Proc. Natl. Acad. Sci. USA 98, 1952-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rissman, E. F., Heck, A. L., Leonard, J. E., Shupnik, M. A. & Gustafsson, J.-A. (2001) Proc. Natl. Acad. Sci. USA 99, 3996-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moggs, J. G. & Orphanides, G. (2001) EMBO Rep. 2, 775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeilschifter, J., Koditz, R., Pfohl, M. & Schatz, H. (2002) Endocr. Rev. 23, 90-119. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson, J. A. & Nilsson, S. (2002) in International Position Paper on Women's Health and Menopause: A Comprehensive Approach, ed. Wenger, N. K. (Natl. Inst. of Health, Bethesda, Publication No. 02-3284), pp. 77-102.