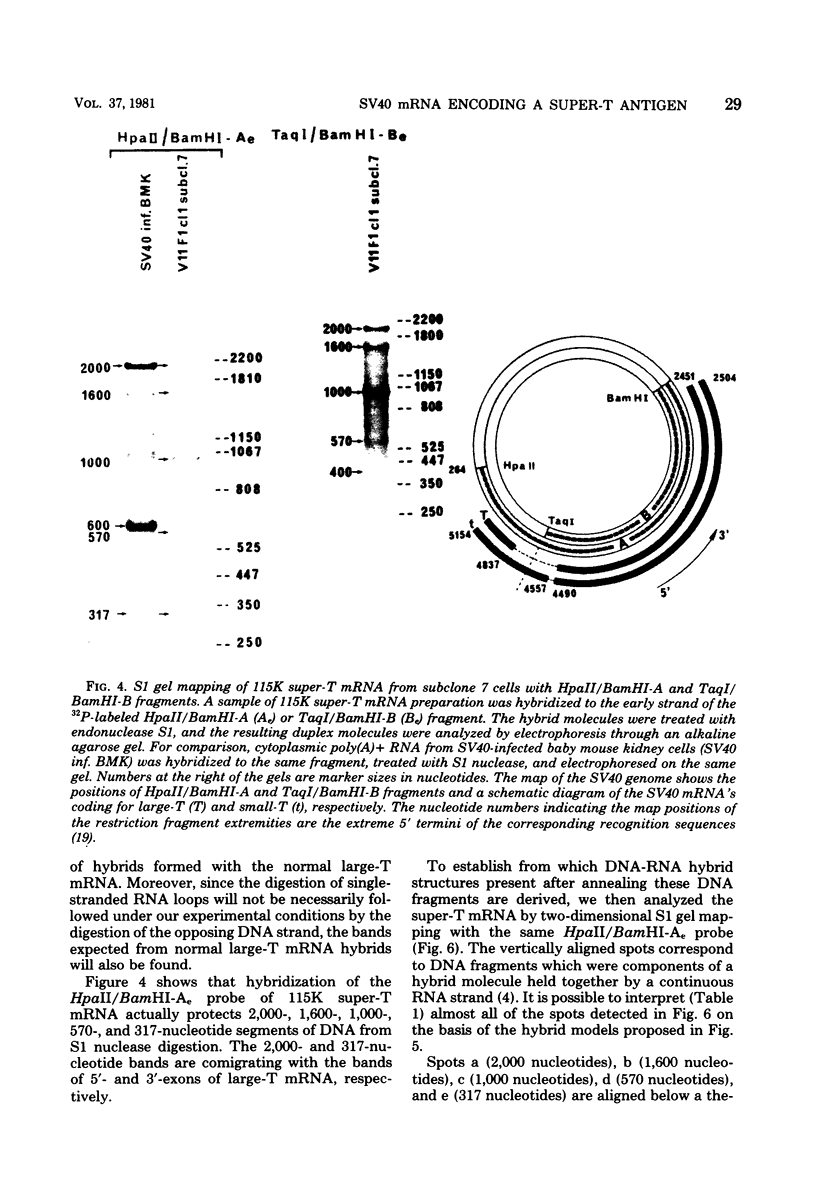
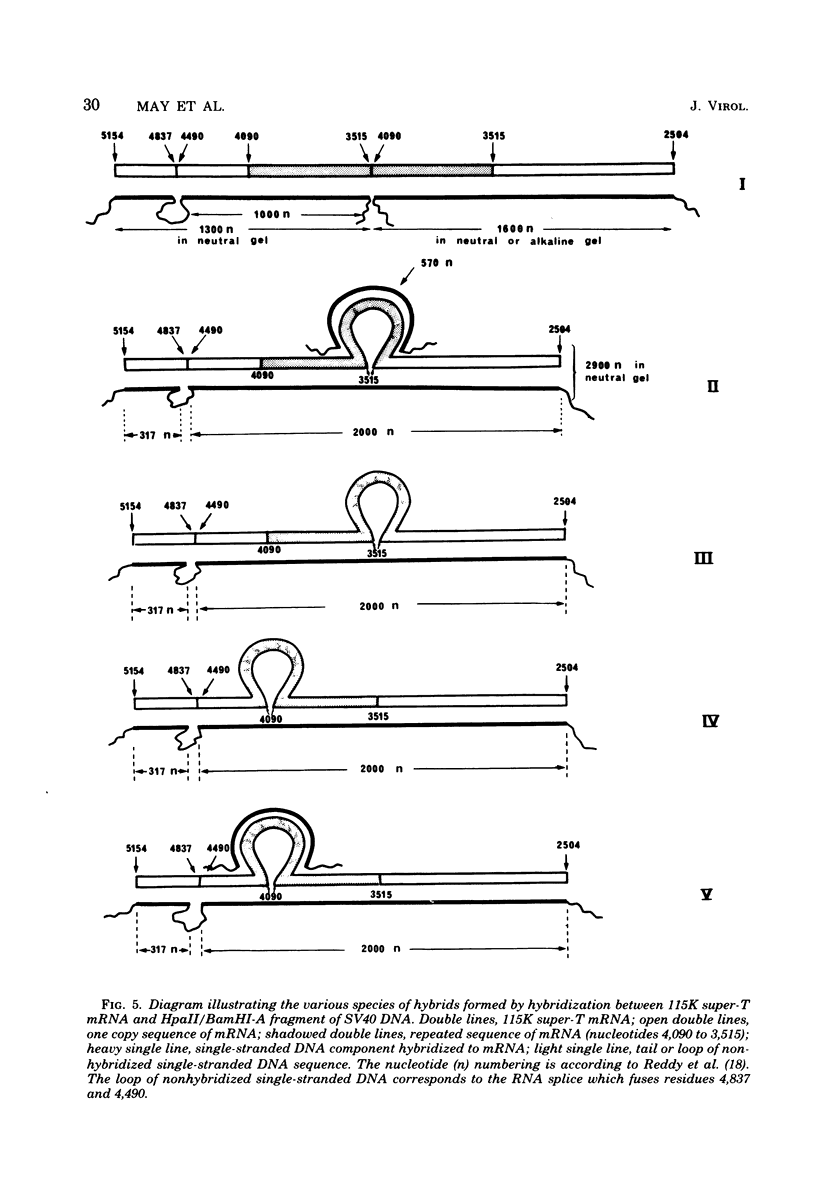
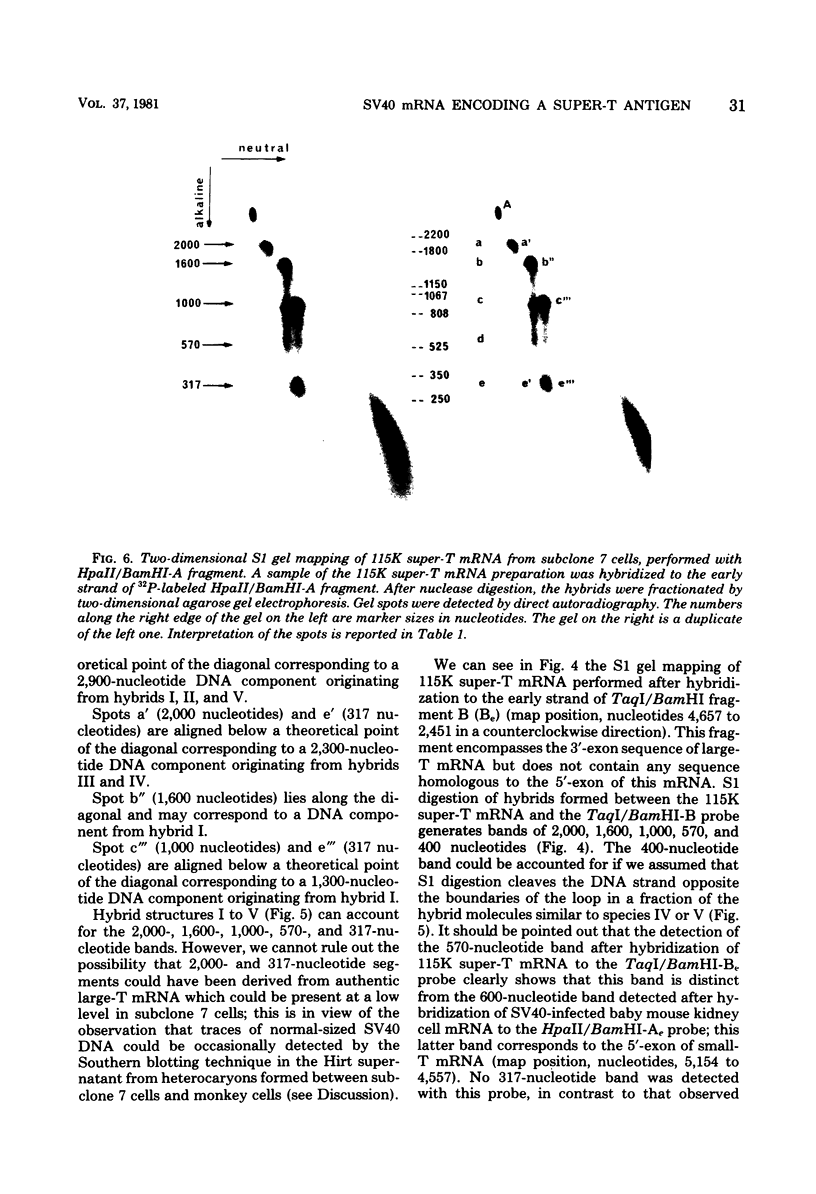
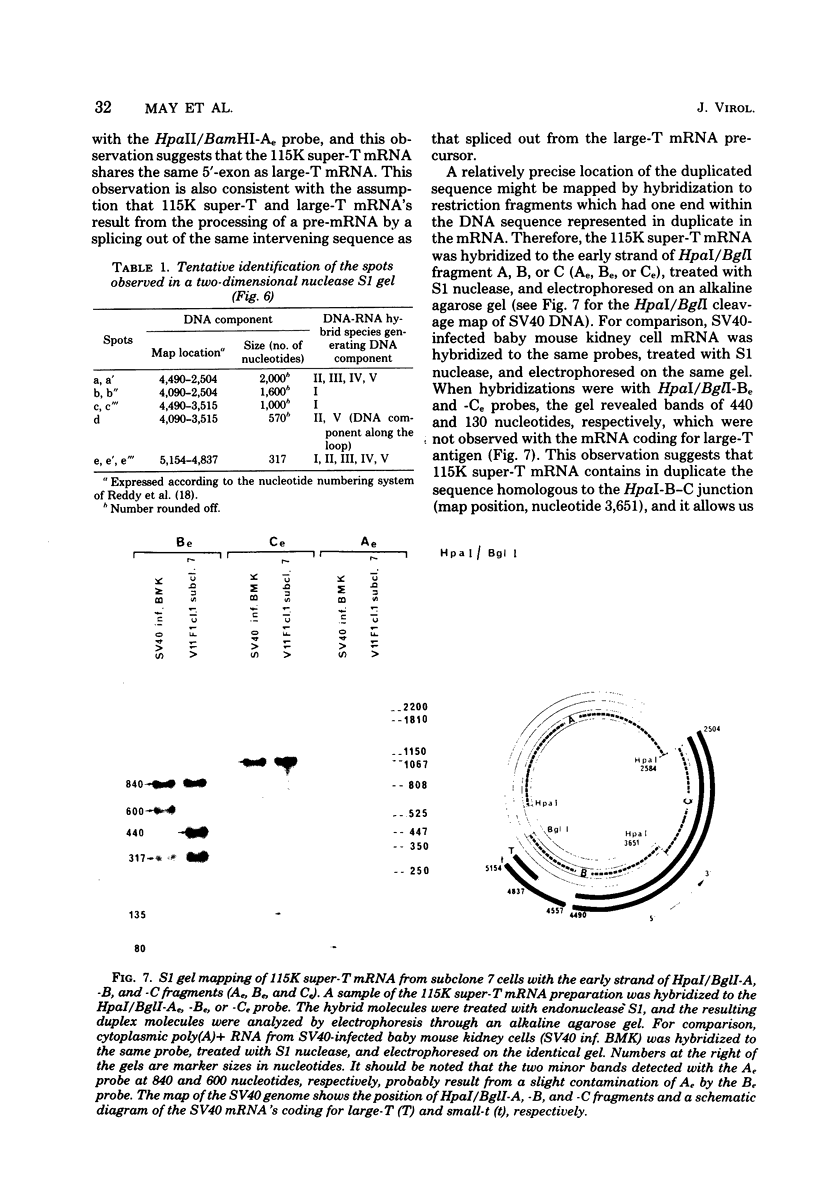
Abstract
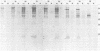
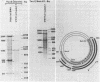
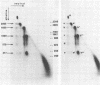
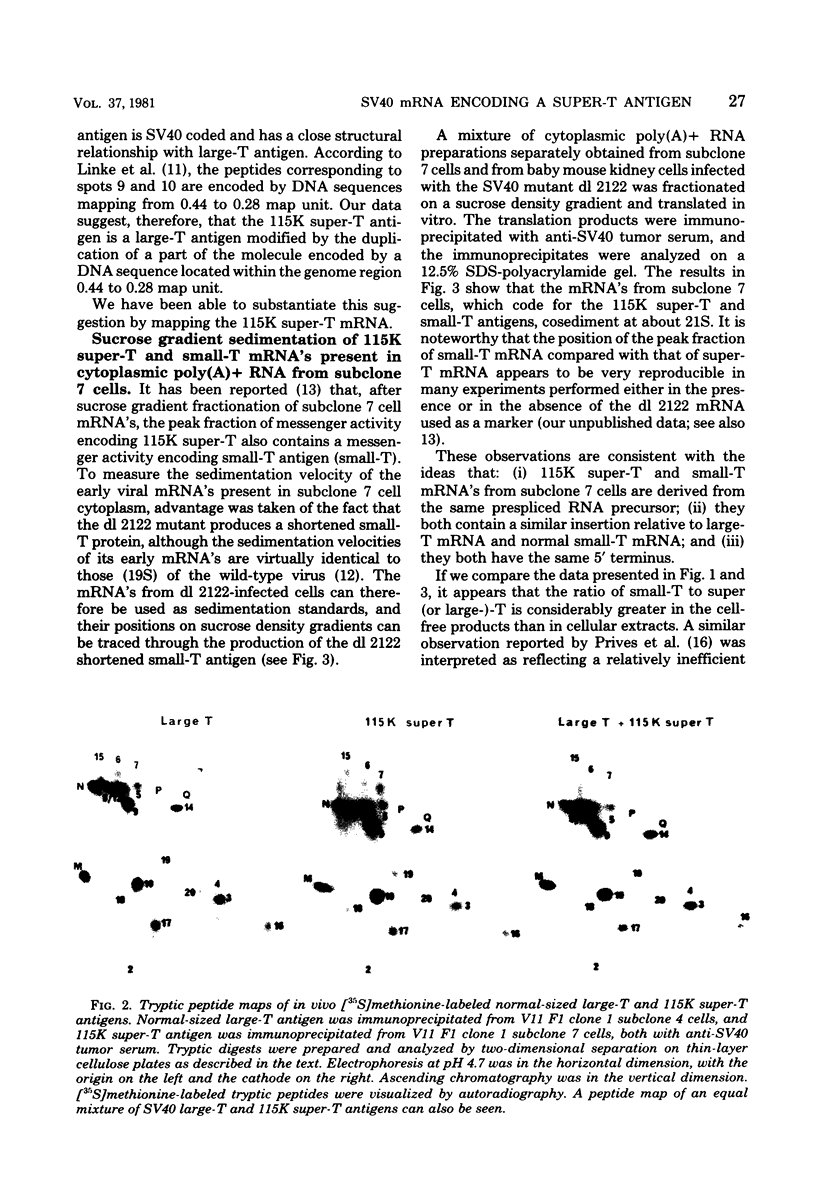
Simian virus 40-transformed V11 F1 clone 1 subclone 7 rat cells produced a considerable amount of an elongated form of large-T antigen with an Mr of 115,000 (115K super-T antigen), but these cells did not produce detectable traces of normal-sized large-T antigen (86,000 daltons) (P. May, M. Kress, M. Lange, and E. May, Cold Spring Harbor Symp. Quant. Biol. 44:189-200, 1980). First, a comparison of the tryptic peptide fingerprints of 115K super-T and large-T antigens suggested that 115K super-T antigen is simian virus 40 coded and contains a duplication of amino acid sequences of large-T antigen. Second, from S1 mapping analysis of 115K super-T mRNA, performed with various restriction fragments of simian virus 40 DNA, it was concluded that super-T mRNA is a form of large-T mRNA containing a tandem duplication of the sequence extending from approximately 0.46 to 0.35 map unit. The duplicated sequence corresponded to that region of the simian virus 40 genome in which 12 of 13 tsA mutation sites are clustered (C. J. Lai and D. Nathans, Virology 66:70-81, 1975).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Simmons D. T., Martin M. A., Mora P. T. Identification and partial characterization of new antigens from simian virus 40-transformed mouse cells. J Virol. 1979 Aug;31(2):463–471. doi: 10.1128/jvi.31.2.463-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Lane D. P., Denhardt D. T., Harlow E. E., Nicklin P. M., Osborn K., Pim D. C. Characterization of the complex between SV40 large T antigen and the 53K host protein in transformed mouse cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):179–187. doi: 10.1101/sqb.1980.044.01.021. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L., Lasne C., Nardeux P., Chouroulinkov I., Monier R. Oncogenic transformation of rat lung epitheloid cells by SV 40 DNA and restriction enzyme fragments. Arch Virol. 1979;62(2):87–100. doi: 10.1007/BF01318062. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol. 1979 Aug;31(2):472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linke H. K., Hunter T., Walter G. Structural relationship between the 100,000- and 17,000- molecular-weight T antigens of simian virus 40 (SV40) as deduced by comparison with the SV40-specific proteins coded by the nondefective adenovirus type 2-SV40 hybrid viruses. J Virol. 1979 Jan;29(1):390–394. doi: 10.1128/jvi.29.1.390-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Kress M., May P. Characterization of two SV40 early mRNAs and evidence for a nuclear "prespliced" RNA species. Nucleic Acids Res. 1978 Sep;5(9):3083–3099. doi: 10.1093/nar/5.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., Kress M., Lange M., May E. New genetic information expressed in SV40-transformed cells: characterization of the 55K proteins and evidence for unusual SV40 mRNAs. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):189–200. doi: 10.1101/sqb.1980.044.01.022. [DOI] [PubMed] [Google Scholar]
- McCormick F., Chaudry F., Harvey R., Smith R., Rigby P. W., Paucha E., Smith A. E. T antigens of SV40-transformed cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):171–178. doi: 10.1101/sqb.1980.044.01.020. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Prives C., Gluzman Y., Winocour E. Cellular and cell-free synthesis of simian virus 40 T-antigens in permissive and transformed cells. J Virol. 1978 Feb;25(2):587–595. doi: 10.1128/jvi.25.2.587-595.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rosenthal L. J. Isolation and characterization of poly(A)-containing polyoma "early" and "late" messenger RNAs. Nucleic Acids Res. 1976 Mar;3(3):661–676. doi: 10.1093/nar/3.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell. 1979 Oct;18(2):335–346. doi: 10.1016/0092-8674(79)90053-9. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Feunteun J., Crawford L. V., Berg P., Fiers W. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J Virol. 1979 Jun;30(3):674–682. doi: 10.1128/jvi.30.3.674-682.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Van de Voorde A., Fiers W. Nucleotide sequence of the simian virus 40 small-t gene. Proc Natl Acad Sci U S A. 1978 May;75(5):2160–2164. doi: 10.1073/pnas.75.5.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]